No safety concerns noted in study of intranasal insulin use

(HealthDay)—Intranasal insulin application appears to be safe, according to a review published online March 6 in Diabetes, Obesity and Metabolism.
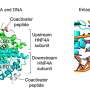
Vera Schmid, from the University of Tübingen in Germany, and colleagues conducted a systematic literature search to identify original research on the use of intranasal human insulin without further additives in humans.
The researchers identified 38 studies (1,092 participants) with acute application and 18 studies (832 participants) with treatment lasting between 21 days and 9.7 years. There were no cases of symptomatic hypoglycemia or severe adverse events identified. After both intranasal insulin and placebo spray, transient local side effects in the nasal area were frequently experienced, but adverse events were less commonly reported. Tests on spray insulin showed it had a chemical stability of up to 57 days.
"Our retrospective review of published studies on intranasal insulin did not reveal any safety concerns. There is, however, insufficient data to ensure long-term safety of this modality of chronic insulin administration," the authors write. "Improved insulin preparations that cause less nasal irritation would be desirable for future treatment."
More information:
Abstract
Full Text (subscription or payment may be required)
Copyright © 2018 HealthDay. All rights reserved.