Medical chemists discover peptic ulcer treatment metallodrug effective in 'taming' superbugs
Antimicrobial resistance caused by "superbugs" is a major public health issue of global concern. Drug-resistant infections kill around 700,000 people worldwide each year. The figure could increase up to 10 million by 2050, exceeding the number of deaths caused by cancers, according to figures of the World Health Organization (WHO).
Current clinical options for treating antibiotic resistant infections include increasing the prescribed antibiotic dose or using a combination therapy of two or more antibiotics. This might potentially lead to further overuse of antibiotics, producing superbugs even more resistant to antibiotics. Nevertheless, the development of antibiotic resistance far outpaces the approval of new antibacterial agents. It takes a decade and costs about $1 billion on average to bring a new drug to market, but bacterial resistance to a new drug only takes a couple of years. Scientists and clinicians need an economical, effective alternative strategy to meet the global public health challenge of antimicrobial resistance.
A research team led by Professor Sun Hongzhe of the Department of Chemistry, Faculty of Science and Dr Richard Kao Yi-Tsun of the Department of Microbiology, Li Ka Shing Faculty of Medicine, the University of Hong Kong (HKU) reports an alternative strategy by repositioning colloidal bismuth subcitrate (CBS), an antimicrobial drug against Helicobacter pylori (H. pylori)-related ulcer.
The bismuth-based metallodrug paralyzes multi-resistant superbugs, e.g. Carbapenem-resistant Enterobacteriaceae (CRE) and Carbapenem-resistant Klebsiella pneumoniae (CRKP) and significantly suppresses the development of antibiotic resistance, allowing the lifespan of currently used antibiotics to be extended. CRE and CRKP can cause deadly infections such as bacteremia, pneumonia and wound infections.
The team is the first to link the "resistance-proof" ability of metallo-drug to the treatment of superbugs. This bismuth drug-based therapy looks set to become the last-line strategy against superbug infections, apart from the development of new antibiotics. Since CBS is a U.S. Food and Drug Administration (FDA)-approved drug, the researchers hope for quick clinical trials.
The findings were published in Nature Communications in January 2018.
CRE is one of the three most dangerous superbugs on the WHO's list of critical priority needs for new antibiotics. CRE resists almost all the clinically available antibiotics and spreads easily through person-to-person contact. If sepsis occurs, the death rate could be as high as 50 percent, according to the Centers for Disease Control (CDC) in the U.S.
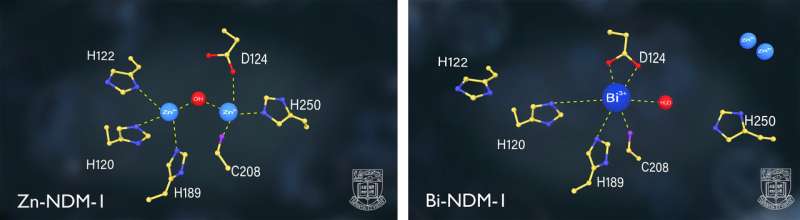
The research team found that CBS as well as other relevant bismuth-based compounds could serve as potent inhibitors of the enzyme NDM-1 (New Delhi metallo-β-lactamase 1), one of the leading resistance determinants changing common bacteria into CRE superbugs. This enzyme arms bacteria with resistance to almost all commonly used beta-lactam antibiotics including the last-resort drug Carbapenem.
The NDM-1 carrying CREs is lethal and extremely difficult to treat, posing a great threat to public health, and may drive the world to the cusp of a "post-antibiotic era." Scientists report that NDM-1 superbugs have now been disseminated to over 70 countries or regions around the world.
Through a series of tests on an NDM-1 Escherichia coli (E. coli) (denoted as NDM-HK), clinically collected by Dr Ho Pak Leung, Director of the HKU Carol Yu Centre for Infection, the team revealed that CBS can halt the superbug, reducing it to a sensitive strain that can be easily killed by commonly used Carbapenem antibiotics. More importantly, the therapy allows the dose of antibiotics to be reduced by 90 percent to attain the same level of effectiveness, and the development of NDM-1 resistance to be slowed down significantly, which will extend the life cycle of currently used antibiotics.
In the mouse model of NDM-1 bacterial infection, combination therapy comprising CBS and Carbapenem significantly prolonged the life expectancy and raised the eventual survival rate of infected mice by more than 25 percent compared to Carbapenem monotherapy. The research team now concentrates on using CBS-based therapy in other animal infection models, e.g. urinary tract infections (UTI), hoping to offer a more extensive approach to combat with antibiotic resistant superbugs.
Dr Ho said, "There is currently no effective approach to overcome the NDM superbug. Bismuth has been used clinically for decades. Knowing that it can tame the NDM is like 'a good rain after a long drought' for the scientific community."
Professor Sun said, "CBS has been clinically used for a long period of time in many countries and regions including Mainland China and Hong Kong, and significantly enhances the eradication rate of resistant H. pylori. Surprisingly, no bismuth-resistant strain has been reported even after a long-term use. We hope CBS-based combination therapy will open up a new horizon for the treatment of infection caused by superbugs, serving as a new and more economical therapy to solve the problem of antimicrobial resistance (AMR)."
More information: Runming Wang et al, Bismuth antimicrobial drugs serve as broad-spectrum metallo-β-lactamase inhibitors, Nature Communications (2018). DOI: 10.1038/s41467-018-02828-6