Defining the brain mosaic in fruit flies and humans
Similar to a mosaic floor where different patterned tiles come together to make a composite and holistic image, our brains consist of billions of unique neurons that connect and generate coordinated brain activity. Unlike a static mosaic, however, our brains are dynamic and activity in the brain changes based on environmental cues. So what makes up the mosaic of the brain? How are individual neurons different from each other? The presence or absence of special types of proteins on individual neurons makes them unique, and the complete range of such proteins on a neuron defines its characteristic ability to respond differentially to particular internal or external stimuli. When individual neurons lose their characteristic protein combination, it can lead to faulty brain activity followed by neurodegeneration diseases and psychiatric disorders. So identifying mechanisms that define which protein is present on which neurons, are of utmost importance.
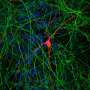
A study published in the journal eNeuro has now shown that across species ranging from fruit flies to humans, neurons in the newly formed brain use a novel form of calcium signaling called store-operated calcium entry (SOCE) to express the correct range of proteins. During SOCE, neurons bring calcium from outside the cell to refill cellular calcium stores depleted by the neuronal response to a range of signals from hormones, neuropeptides, and even neurotransmitters. The function of SOCE in neurons has been difficult to understand primarily because neurons have several alternate mechanisms of calcium entry. We have used targeted genetic approaches to study how SOCE affects neuronal function in Drosophila neurons, and more recently in neuronal precursor cells derived from human stem cells.
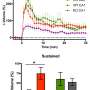
The fruit fly, like many other insects, grows from a crawling larva to a sessile pupa in which the larval brain is re-modeled for life as a fly. Inside the apparently dormant pupa, however, neurons that are being remodeled are busy making the right connections with other neurons—a process called neural circuit development. When proteins required for calcium signaling were removed specifically from neurons of the flight circuit, flies that emerged from pupae were unable to fly for long. By comparing brains from such poorly flying flies with normal flies, it became clear that specific proteins that help neurons talk to each other were present at much lower levels when calcium signaling is impaired in the developing flight circuit of a fruit fly pupa.
Earlier work showed that genes encoding these proteins were themselves expressed at lower levels when calcium signaling was impaired. Replacing them in a fruit fly with impaired calcium signaling allowed for much better flight. In these "rescued" flies, the ability of flight neurons to talk to each other, a process called neurotransmission, also improved.
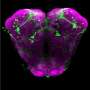
A majority of cellular and molecular processes are conserved in organisms separated from each other across large evolutionary timescales. Thus, studies of the fruit fly have illustrated aspects of human development, behavior and disease. Because direct experiments of gene expression in human brains are not possible, the researchers chose instead to test this idea in human neural precursor cells derived from human stem cells. A loss of a particular class of calcium signaling led to altered gene and protein expression. As shown in our recently published article in Frontiers in Molecular Neuroscience, the neural precursor cells became more neuron-like at an earlier stage, suggesting that calcium signaling during development helps in expression of proteins required to maintain neural precursor cells in a state where they can divide. This finding is potentially important for understanding disease syndromes that affect normal growth of the human brain.
Our findings from the fruit fly and human neural precursor cells show that the same calcium signaling mechanism is required for generating specific classes of neurons in the brain. In the fruit fly, we know that loss of such signaling affects their ability to fly normally. In the human brain, it is potentially implicated in growth at earlier stages of brain formation. Does the loss of such calcium signaling in adult human neurons also change their function and affect human health? Work from other labs suggests that this might be the case. For example, in the human neurodegenerative disorder spinocerebellar ataxia, calcium signaling is reduced due to inherited mutations. Is this due to changes in gene expression in the affected neurons over time? The researchers hope to answer that question in future experiments.
More information: Renjitha Gopurappilly et al. Stable STIM1 Knockdown in Self-Renewing Human Neural Precursors Promotes Premature Neural Differentiation, Frontiers in Molecular Neuroscience (2018). DOI: 10.3389/fnmol.2018.00178
Shlesha Richhariya et al. dSTIM- and Ral/Exocyst-Mediated Synaptic Release from Pupal Dopaminergic Neurons Sustains Drosophila Flight, eneuro (2018). DOI: 10.1523/eneuro.0455-17.2018