Upgrading the toolbox for Duchenne muscular dystrophy research with a new rabbit model
Scientists rely on the use of animal models to improve our understanding of lethal muscle-wasting disease Duchenne muscular dystrophy (DMD), and to develop safe new therapies. A newly developed rabbit model, created through the use of CRISPR/Cas-9 genome editing, exhibits greater clinical similarity to human patients than the mouse models currently in use, with huge potential to advance DMD research.
The muscle weakness that defines DMD first manifests in the hips and pelvis of affected boys at around four years old. This X-linked recessive disease affects around 1 in 3500 live male births and is caused by a defective gene disrupting production of a protein called dystrophin, which is essential for keeping muscle fibres intact. As the disease develops, muscle loss progresses from upper legs to upper arms and shoulders, leading to increased difficulty in standing up, climbing stairs and walking. By the time the boys reach their early teens, DMD also begins to affect the respiratory muscles and heart, which until recently meant that affected individuals did not survive much beyond their teen years. Improved medical care means that life expectancy has increased, with survival into the early 30s or even 40s becoming more common. However, there is still no cure and most current treatments focus on slowing and controlling onset of symptoms through drugs such as a steroids, physical therapy and orthopaedic appliances to improve mobility.
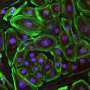
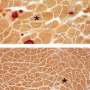
New gene therapy treatments that will target the affected gene to allow production of some level of functional dystrophin are under development, with a small number of drugs already commercially available. However, the available drugs target only a small percentage of the different dystrophin gene mutations that cause DMD. An important part of research into the physiological causes and testing of new therapies for any disease is the use of animal models. Previously, the vast majority of research into DMD has been conducted in mice, but despite a large number of potential treatments having been identified using mouse models, physiological differences between mice and humans means that relatively few have been translated to successful clinical trials. Dog models replicate human DMD better than mice as their physiology is more similar, and pig models for DMD are also under development. However, although large animal models have great potential for increasing our understanding of DMD and the development of new therapies, their use is expensive and limited to only a few facilities around the world. To fill the current gaps in animal models available for DMD research, new research published in open access journal Disease Models & Mechanisms reveals a novel rabbit model, created through CRISPR/Cas9 embryonic genome editing, by a team of scientists based at Jilin University, China and The Ohio State University Wexner Medical Center, USA. Crucially for successful DMD research and potential translation to preclinical studies and human clinical trials, the rabbits exhibit many of the same typical signs of muscular dystrophy as DMD patients, including severely impaired physical activity, elevated serum creatine kinase levels, progressive muscle loss and damage to heart muscle.
Accelerating developments with CRISPR/Cas9
Key to the development of this animal model was the CRISPR/Cas9 genome editing technology that has revolutionised many aspects of modern biomedical research. Lead researcher Dr. Renzhi Han explains that "While it used to take two to three years to generate a knockout mouse model, now with CRISPR/Cas9, it takes only months to generate knockout models of various species such as mice, rats, rabbits, dogs, pigs and even monkeys." Prior to genome editing technology, approaches to creating animal models involved turning off the entire gene linked to disease in humans. However, in many cases the mutation causing the disease involves only subtle changes to the gene. This means that disease pathogenesis may be specific to these individual mutations, and may not be well modelled simply by turning off the whole gene. Precise nucleotide manipulation with CRISPR-derived approaches allows the development of animal models that more accurately reflect the disease-causing mutation, and allows them to be developed within a much shorter time frame. In the most commonly used mouse model of DMD, disruption of the dystrophin gene occurs at a specific site, exon 23, resulting in loss of the protein. Using CRISPR/Cas9, Dr. Han and colleagues were able to specifically target a different region, exon 51, that is a common mutation 'hotspot' in the human gene. Paired with a physiology that is more similar to that of humans, this means that the rabbit model more closely replicates the dystrophin deficiency of human DMD.
Advancing the search for a breakthrough
Looking to the future, Dr. Han reflects, "We believe a better model will facilitate studies to advance the understanding of DMD and aid the development of novel therapies." Currently, the scientists are focused on developing novel therapeutic strategies for muscular dystrophies. Their recent research has demonstrated that CRISPR/Cas9 genome editing therapy can restore dystrophin expression and cardiac function in dystrophic mice. With the availability of the DMD rabbit model, the team plan to collaborate with other labs to further test the feasibility of this and other therapeutic strategies, pushing for a breakthrough in DMD research.
More information: Tingting Sui et al, A novel rabbit model of Duchenne muscular dystrophy generated by CRISPR/Cas9, Disease Models & Mechanisms (2018). DOI: 10.1242/dmm.032201