New approaches for drug-based kidney disease therapy

Whilst most scientific attention in the fight against kidney disease is currently focused on stem cells, the EVESTIMINJURY project has been investigating the extracellular vesicles being released by these stem cells. The resulting treatment could provide an excellent alternative to cell-based therapy.
A few years ago, scientists found out just how amazing our kidneys actually were: contrary to popular belief, they had the capacity to regenerate and repair themselves throughout life. This regeneration process, however, has its limitations. In the face of the absence of symptoms during the early stages of kidney disease and a frequent lack of follow-up, it hasn't been enough to prevent many patients across Europe from permanently losing some kidney functions or even dying from chronic and acute kidney disease.
It's easy to understand why this context would make stem cells look like the holy grail. Clinical trials for several types of stem cells are ongoing and show much promise. But according to the five organisations responsible for the EVENTEMINJURY project, the real potential doesn't lie in the stem cells themselves, but rather in the extracellular vesicles (EVs) released by these stem cells.
"EVs have been shown to mimic the biological activity of the cells by transferring stem cell-derived molecules (proteins, biological active lipids and nucleic acids) able to activate endogenous processes to the injured tissue. In patients with acute and chronic kidney diseases, EVs may be an alternative to cell-based therapy, with the advantage that EVs derived from stem cells are not immunogenic, are bio-compatible and can be administered as a drug," explains Professor Fiorella Altruda, who coordinated the project on behalf of Bioindustry Park 'Silvano Fumero'.
This potential had already been investigated prior to the project, with a focus on acute kidney injury. The studies were a source of optimism, but what really caught the eye of Prof. Altruda and her colleagues was the lack of information available on the molecular mechanisms at play.
The EVESTIMINJURY project was meant to fill this gap. It provides mechanistic information on EV regenerative mechanisms and, more specifically, the potential role of EVs in chronic kidney injury by studying the ability of stem cell-derived EVs to inhibit fibrosis.
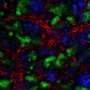
Different subsets of EVs were characterized with regards to their anti-fibrotic properties. The project consortium used three different separation techniques for that, to isolate EVs from bone marrow mesenchymal stem cells (MSC) – the most widely used cells for the treatment of kidney diseases – and hepatic liver stem cells (HLSC).
"The first isolation technique was based on differential ultracentrifugation," explains Prof. Altruda. "It allowed for the separation of two main fractions at 10K and 100K g. Then, the second protocol was based on the selection of different EV sub-populations using the flotation iodixanol gradient separation method, which enabled the selection of 12 fractions containing different subsets of EVs. Finally, the third isolation technique – based on size exclusion chromatography – enabled the separation of a pure exosomal fraction from other EV subsets and proteins. EV dimension and profile were analyzed using Nanosight LM10 from project partner NanoSight Ltd, and EVs were also characterized by western blot analysis, electron microscopy and cytofluorimetric analysis."
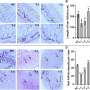
The results demonstrate how fractions differ in terms of molecular components as well as in vitro/in vivo regenerative potential. The total EV fraction was shown to be the most effective in inducing tubular epithelial regeneration and anti-fibrotic effect both in vitro and in vivo. In particular, pre-clinical studies confirmed that stem cell-derived EVs display a significant pro-regenerative effect on the kidney.
Buoyed by this success, the consortium will now focus on the up-scaling of EV production from stem cells as well as a GMP protocol for potential clinical use. All knowledge gathered throughout the project will be made available to other organisations and universities.
"Of course, using this knowledge to start curing patients will require more time. Further studies are needed to investigate bio-distribution, bio-availability, pharmaco-dynamics, bio-safety, as well as how and when to administer the drug. Moreover, the criteria for the characterisation of a product for human use are still to be defined and approved by regulatory agencies," Prof. Altruda says.