Innovative CRISPR study recreates genetic steps required for benign moles to turn malignant
UC San Francisco researchers have identified the sequence of genetic changes that transform benign moles into malignant melanoma in a large cohort of human skin cancer patients and have used CRISPR gene editing to recreate the steps of melanoma evolution one by one in normal human skin cells in the lab. The research identified key molecular warning signs that could be used by clinicians to catch developing cancers before they spread and could also lead to new targeted therapies.
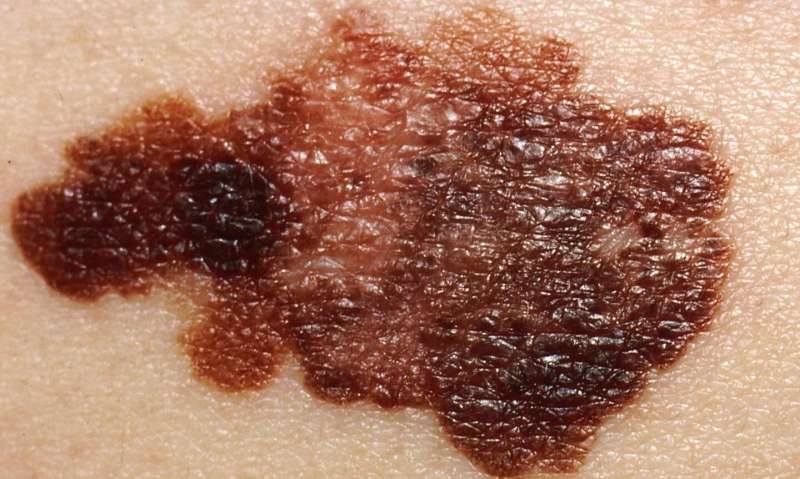
Melanoma is most commonly triggered by exposure to ultraviolet (UV) rays in sunlight, which damages DNA, creating genetic mutations that cause skin cells to multiply and spread. Often, these cells produce benign moles, pigmented skin growths that are self-limiting in size. While most moles never turn cancerous, some can transform into malignant melanoma and rapidly spread to other parts of the body. Melanomas can almost always be successfully treated if they are caught early, but fewer than 30 percent of patients with metastatic melanoma survive more than five years, making melanoma the deadliest form of skin cancer.
The new research, published July 9, 2018, in two companion papers in Cancer Cell, for the first time systematically traced how new mutations and changes in gene activity break down cellular protective mechanisms, allowing moles to transform into melanoma and begin to spread. The new studies also identify new biomarkers that could be used in the clinic to more reliably detect rapidly progressing melanomas that require additional treatment beyond surgical removal.
Dermatologists currently diagnose melanomas by taking biopsy samples of suspicious skin growths. If the lesion turns out to be a melanoma, pathologists measure its thickness to determine how far it has progressed and whether surgery alone will be enough to eliminate the cancer: The thicker the melanoma, the greater the risk that it has already begun spreading to other parts of the body. But this approach is not very reliable—some thin melanomas metastasize and some thick ones do not.
"It's a very crude assessment of the progression state of a tumor to measure it with a ruler. We'd prefer to be able to measure a mole's genetic state to assess its risk of turning malignant, but the biology of this transformation has not been fully understood," said Boris Bastian, MD, Ph.D., a UCSF Health skin cancer pathologist who directs the Clinical Cancer Genomics Laboratory for the UCSF Helen Diller Comprehensive Cancer Center and was one of the leaders of the new research.
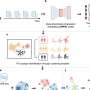
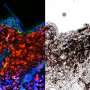
In the first study, led by Bastian and UCSF cancer geneticist Hunter Shain, Ph.D., the researchers studied a unique dataset surgically removed melanoma tissue samples from 82 patients in which malignant tumors and the benign moles from which they had developed were preserved side-by-side. In a subset of patients, the researchers also obtained matched samples of metastatic tumors and the primary melanomas in the skin from which the metastatic colonies had derived. By analyzing these 230 tissue samples, the researchers were able to compare molecular differences between benign growths, malignant melanomas, and metastatic colonies within the same patients.
The researchers sequenced tumor DNA to identify gene mutations arising at different stages of cancer evolution and also measured changes in RNA to connect these mutations to related changes in gene activity. "This is the first study to profile both DNA and RNA from matching melanoma samples and precursor moles from the same patients," Shain said.
"This was made possible by a close collaboration with the large UCSF Health skin pathology service," Bastian added. "Hybrid samples where you can see both the melanoma and the mole that it grew out of are not that common—but from the thousands of melanoma cases we diagnose, we were able to select suitable cases that allowed us to study how the cancer evolves."
The researchers found that as tumors progress, multiple independent gene mutations repeatedly tweak central molecular pathways controlling cell growth, tumor suppression and DNA regulation until enough mutations accumulate to break down cells' natural protective mechanisms and trigger cancer.
"The field has a tendency to oversimplify how cancers evolve, as if there were just a switch that gets flipped on." Shain said, citing the team's own previous work pointing to early activation of the MAP kinase pathway in melanomas. "Now we see that this pathway is turned on just a little early on, then ramped up over the course of tumor evolution. We think this may allow cancers to avoid cellular alarm bells until enough genetic changes have accumulated that the alarms no longer function."
Among other findings, the researchers found that moles consistently developed mutations in the SWI/SNF class of DNA regulatory genes as a key step in their transition to malignancy, suggesting this marker could be used clinically to identify dangerous moles in need of treatment.
In the second paper, led by UCSF melanoma geneticist Robert Judson, Ph.D., the researchers performed an innovative experiment to recreate and study the steps of melanoma evolution in a controlled laboratory setting. Using CRISPR-Cas9 gene editing, the scientists precisely inserted the sequence of mutations they had observed in clinical specimens into normal human skin cells in the lab.
This allowed the researchers to examine the specific biological effects of each step in melanomas' evolution: for example, they were surprised to discover that mutations disrupting the central tumor suppressor gene CDKN2A did not simply unleash tumor growth, as had been predicted, but also caused affected cells to become highly mobile (and therefore more prone to invade and spread to other parts of the body) through activation of a transcription factor protein called BRN2.
In addition to identifying a key mechanism driving melanoma's deadly ability to metastasize, the new research demonstrates a novel use for CRISPR gene-editing as a laboratory tool for studying melanoma.
"Previous studies have typically used cell lines derived from advanced-stage cancers, where there is so much going wrong at the genetic level that it's hard to know what's causing what," Judson said. "Here we're looking at otherwise healthy skin cells with specific mutations engineered in. It's much clearer what each mutation does."
"If you go into a cell line bank, you'll never find a mole cell line," Shain added. "Rob's approach allowed us to study the underlying biology of melanoma precursors better than ever before."
The researchers hope this new tool and the discoveries it has already produced will significantly speed research into how to more rapidly diagnose and treat melanoma patients. In particular, they hope that their discoveries will be rapidly incorporated into clinical genetic tests that will allow physicians to quickly assess patients' risk of skin cancer and assign those at highest risk to early treatment.
"Because we haven't had the tools to identify who is at high risk of metastasis, we have tended to treat everybody as if they were at high risk, exposing them to intense treatment and potential side effects," Bastian said. "We anticipate that looking at genetic changes in melanoma samples here at UCSF will allow us to identify melanomas that have progressed to a dangerous state and deploy systemic treatments earlier, which will increase their effectiveness for our patients."