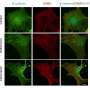
Cellular changes lead to chronic allergic inflammation in the sinus

Chronic rhinosinusitis is distinct from your average case of seasonal allergies. It causes the sinuses to become inflamed and swollen for months to years at a time, leading to difficulty breathing and other symptoms that make patients feel miserable. In some people, this condition also produces tissue outgrowths known as nasal polyps, which, when severe enough, have to be removed surgically.
By performing a genome-wide analysis of thousands of single cells from human patients, MIT and Brigham and Women's Hospital researchers have created the first global cellular map of a human barrier tissue during inflammation. Analysis of this data led them to propose a novel mechanism that may explain what sustains chronic rhinosinusitis.
Their findings also offer an explanation for why some rhinosinusitis patients develop nasal polyps, which arise from epithelial cells that line the respiratory tract. Furthermore, their study may have broader implications for how researchers think about and treat other chronic inflammatory diseases of barrier tissues, such as asthma, eczema, and inflammatory bowel disease.
"We saw major gene-expression differences in subsets of epithelial cells which had been previously obscured in bulk tissue analyses," says Alex K. Shalek, the Pfizer-Laubach Career Development Assistant Professor of Chemistry, a core member of MIT's Institute for Medical Engineering and Science (IMES), and an extramural member of the Koch Institute for Integrative Cancer Research, as well as an associate member of the Ragon and Broad Institutes.
"When you look across the entire transcriptome, comparing cells from patients with different disease statuses over thousands of genes, you can start to understand the relationships between them and discover which transcriptional programs have supplanted the usual ones," Shalek says.
The lead authors of the paper, which appears in the Aug. 22 issue of Nature, are Jose Ordovas-Montanes, an IMES postdoc fellow supported by the Damon Runyon Cancer Research Foundation, and Daniel Dwyer, a research fellow at Brigham and Women's Hospital. Shalek and Nora Barrett, an assistant professor of medicine at Brigham and Women's, are the paper's senior authors.
Clinical single-cell RNA sequencing
Last year, Shalek and his colleagues developed a new portable technology that enables rapid sequencing of the RNA contents of several thousand single cells in parallel from tiny clinical samples. This technology, known as Seq-Well, allows researchers to see what transcriptional programs are turned on inside individual cells, giving them insight into the identities and functions of those cells.
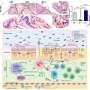
In their latest study, the MIT and Brigham and Women's researchers applied this technology to cells from the upper respiratory tract of patients suffering from chronic rhinosinusitis, with the hypothesis that distinct gene-expression patterns within epithelial cells might reveal why some patients develop nasal polyps while others do not.
This analysis revealed striking differences in the genes expressed in basal epithelial cells (a type of tissue stem cell) from patients with and without nasal polyps. In nonpolyp patients and in healthy people, these cells normally form a flat base layer of tissue that coats the inside of the nasal passages. In patients with polyps, these cells begin to pile up and form thicker layers instead of differentiating into epithelial cell subsets needed for host defense.
This type of gross tissue abnormality has been observed through histology for decades, but the new study revealed that basal cells from patients with polyps had turned on a specific program of gene expression that explains their blunted differentiation trajectory. This program appears to be sustained directly by IL-4 and IL-13, immune response cytokines known to drive allergic inflammation when overproduced at pathologic levels.
The researchers found that these basal cells also retain a "memory" of their exposure to IL-4 and IL-13: When they removed basal cells from nonpolyps and polyps, grew them in equivalent conditions for a month, and then exposed them to IL-4 and IL-13, they found that unstimulated cells from patients with polyps already expressed many of the genes that were induced in those without polyps. Among the IL-4 and IL-13 responsive memory signatures were genes from a cell signaling pathway known as Wnt, which controls cell differentiation.
Immunologists have long known that B cells and T cells can store memory of an allergen that they have been exposed to, which partly explains why the immune system may overreact the next time the same allergen is encountered. However, the new finding suggests that basal cells also contribute a great deal to this memory.
Since basal cells are stem cells that generate the other cells found in the respiratory epithelium, this memory may influence their subsequent patterns of gene expression and ability to generate mature specialized epithelial cells. The team noted a substantial impact on the balance of cell types within the epithelium in patients with severe disease, leading to a population of cells with diminished diversity.
"Once you know that IL-4 and IL-13 act on stem cells, it changes the way in which you have to think about intervening, versus if they acted on differentiated cells, because you have to erase that memory in order to bring the system back to homeostasis," Shalek says. "Otherwise you're not actually dealing with a root cause of the problem."
Blocking cytokines in humans
The findings suggested that ongoing efforts to block the effects of IL-4 and IL-13 might be a good way to try to treat chronic rhinosinusitis, a hypothesis that the researchers validated using an antibody that blocks a common receptor for these two cytokines. This antibody has been approved to treat eczema and is undergoing further testing for other uses. The researchers analyzed the gene expression of basal cells taken from one of the patients with polyps before and after he had been treated with this antibody. They found that most, but not all, of the genes that had been stimulated by IL-4 and IL-13 had returned to normal expression levels.
"It suggests that blockade of IL-4 and IL-13 can help to restore basal cells and secretory cells towards a healthier state," Ordovas-Montanes says. "However, there's still some residual genetic signature left. So now the question will be, how do you intelligently target that remainder?"
The researchers now plan to further detail the molecular mechanisms of how basal cells store inflammatory memory, which could help them to discover additional drug targets. They are also studying inflammatory diseases that affect other parts of the body, such as inflammatory bowel disease, where inflammation often leads to polyps that can become cancerous. Investigating whether stem cells in the gut might also remember immunological events, sustain disease, and play a role in tumor formation, will be key to designing early interventions for inflammation-induced cancers.
More information: Allergic inflammatory memory in human respiratory epithelial progenitor cells, Nature (2018). DOI: 10.1038/s41586-018-0449-8 , www.nature.com/articles/s41586-018-0449-8