Scientists sweep cellular neighborhoods where Zika hides out
Most people infected with Zika never show symptoms. But the virus sometimes causes severe disability—from microcephaly in babies to weakness or partial paralysis in adults—and there is no treatment. In a recent study in the journal Molecular & Cellular Proteomics, researchers report a comprehensive study of how the virus interacts with host cells. One of their findings offers insight into how Zika escapes immune signaling and proliferates inside the body.
Like most viruses, Zika accomplishes a lot with only a few tools. It has just one protein coding gene, which produces a single polypeptide that's cleaved into 10 smaller proteins—a number dwarfed by the estimated 20,000 protein-coding genes in a human cell. Nevertheless, Zika can take over the vastly more complex human cell, repurposing it into a virus factory. Brian Raught, a researcher at the University of Toronto, said he found that process fascinating.
"With just these 10 proteins, this crazy virus turns your cells into zombies that do its bidding," said Raught. "I always found that mindblowing."
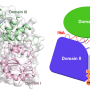
Researchers in Raught's lab, led by postdoctoral fellow Etienne Coyaud, wanted to find out how the handful of Zika proteins were able to hijack the host cell. They knew that the feat must depend on physical interactions between viral proteins and proteins native to the cell—but which ones?
Because of the increasing evidence linking Zika infections in expectant mothers to microcephaly in their children, Raught said, "We thought it better to leave the actual virus work to the experts." Instead of using infectious material, the team made 10 strains of human cells—each expressing one of Zika's 10 proteins. By adding a small "epitope" tag to each viral protein (called a "Flag" tag), they were able to retrieve the viral proteins using an antibody that binds to this tag. The host proteins that stuck tightly to each viral protein came along for the ride; the researchers used a technique called mass spectrometry to identify those human proteins.
But there was a drawback with this approach. What if proteins from the human cell were interacting with Zika proteins, but separating from them before being extracted? To identify proteins that are close to, but not inseparably intertwined with, each viral protein, the team used a second technique called proximity labeling. In essence, they rigged each viral protein with an enzyme that would attach the sticky biotin tag onto anything that came close enough.
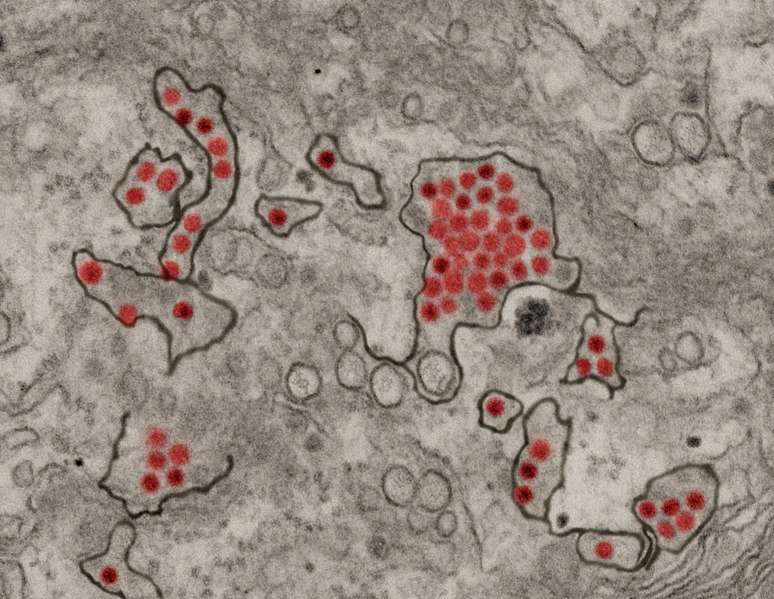
Proximity labeling is especially useful for detecting interactions with proteins embedded in cell membranes; those molecules are notoriously difficult to isolate. "This is a really big asset of a proximity-labeling approach compared to traditional approaches," said Coyaud. Because Zika, like many viruses, enters the cell in a membrane-bound envelope, and reorganizes many of its host's membrane-bound organelles in the course of infection, its interactions with membrane proteins might be key to understanding the viral life cycle.
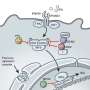
When the researchers combined the results of the two approaches, they could piece together a detailed picture of what Raught calls the "neighborhood" within the cell where each Zika protein takes up residence.
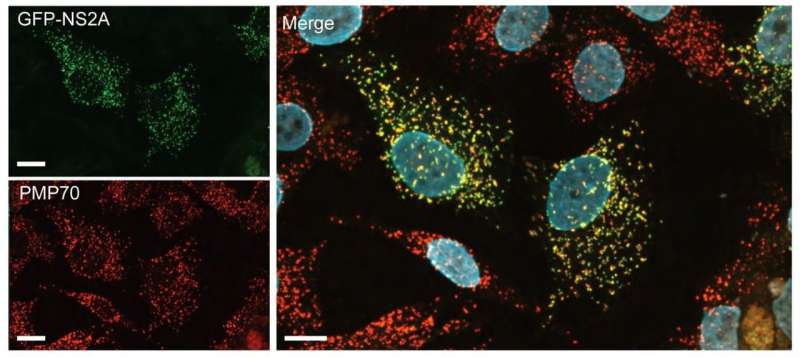
For example, the researchers found that one Zika protein, called NS2A, interacted with many human neighbors in a host organelle called the peroxisome. Peroxisomes are involved in innate immune signaling, a type of early-warning alarm that goes off when viruses are present. Proteins from Dengue and West Nile viruses, which are in the same family as Zika, have been shown to associate with peroxisomes and disrupt antiviral signaling.
Using fluorescence microscopy, said Raught, "we could see that this one protein (NS2A) only localized to peroxisomes." Because NS2A is thought to be involved in replication of the virus's genome and construction of its protein shell, its preference for the peroxisome led the team to investigate whether the peroxisome might be involved in replication. The team recruited some virologists with access to a biocontainment facility, who could test whether infectious Zika virus can proliferate in cells without peroxisomes. Turns out, in those cells, the virus proliferates more slowly.
"There really does appear to be an important link between having a peroxisome and being able to efficiently make Zika virus," said Raught. "We don't know exactly why that is." But the host-virus protein interaction data showed them where to look.
The dataset Raught's lab has collected is full of such clues. Slightly more than half of the human proteins the researchers found interacting with viral proteins are known to be involved in other viral infections, while the rest had not yet been described. According to Raught, "a better understanding of these processes will allow us to identify specific vulnerabilities in the virus life cycle, where antiviral drugs can be targeted."
More information: Etienne Coyaud et al, Global interactomics uncovers extensive organellar targeting by Zika virus, Molecular & Cellular Proteomics (2018). DOI: 10.1074/mcp.TIR118.000800