Duchenne muscular dystrophy: How muscle cells journey to the dark side

Promoting repair of dystrophic muscles is a major goal in the treatment of muscular dystrophies but is complicated by the incomplete knowledge of the cellular and molecular events that drive muscle regeneration.
Answers could lie in better understanding muscle repair—which resembles a delicate cellular dance choreographed by special cells called fibro-adipogenic progenitors (FAPs). Researchers already know these cells have a dark side—they are also responsible for the muscle wasting and scarring that occurs during Duchenne muscular dystrophy (DMD).
Now, scientists at Sanford Burnham Prebys Medical Discovery Institute (SBP) have revealed that FAPs don't have just one identity—but several distinct identities that emerge during key stages of muscle regeneration. Importantly, the FAPs that drive the symptoms of DMD have defined markers, meaning they could be targeted for drug development. The study was published in Nature Communications.
"There is increasing evidence from mouse studies that FAPs may play a critical role in muscle regeneration," says Grace Pavlath, Ph.D., senior vice president and scientific program director of the Muscular Dystrophy Association (MDA). "This study provides further insight into the mechanisms underpinning impaired regeneration and the development of fibrosis in DMD, and suggests future avenues for therapeutic intervention."
DMD mostly affects boys and is caused by the absence of a muscle-strengthening protein called dystrophin. Over time, muscle is replaced by scar tissue and fat, a process called fibrosis that ultimately leads to muscle wasting and weakness. Most people with DMD do not survive past their mid-20s.
"While advances are being made, there is still an urgent need for effective treatments for DMD," says Pier Lorenzo Puri, M.D., senior author of the study; professor in the Development, Aging and Regeneration Program at SBP; and lab director at Fondazione Santa Lucia IRCCS. "This discovery reveals novel cell targets for selective interventions that may promote regeneration and prevent fibrosis in DMD muscles."
Adds Filippo Buccella, founder of the Duchenne Parent Project Italy, part of an international federation created by parents to accelerate the development of new therapies, "This major advancement sheds a new light on the complex process of muscle degeneration/regeneration and may indeed improve the lives of Duchenne patients and their families. This breakthrough comes years after working with skilled physicians and great scientists like Dr. Puri, and it will be invaluable for the many patients and families who as of today are involved worldwide with experimental clinical trials."
Mapping FAPs over time
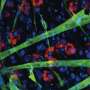
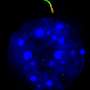
Puri's team analyzed the transcriptome of single FAP cells, which shows the genes that are turned on or off, from samples of muscle tissue obtained from mouse models of acute injury and DMD. This work identified cellular markers unique to a subpopulation of FAPs (sub-FAPs).
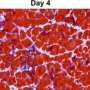
The scientists then applied the transcriptome analysis to each of the identified sub-FAPs to track the relative amounts of gene expression and types of genes expressed in three settings of muscle regeneration: following acute injury; during DMD; and immediately after birth, which uses a different regeneration process from adult muscle repair.
Clear patterns emerged and revealed that the identified sub-FAPs transitioned through different functional states—correlating with key events during the muscle regeneration process. At early stages after acute injury, sub-FAPs expressing the cell surface marker Tie2 appear. They were followed by transient sub-FAPs expressing the cell surface marker Vcam1. Genome-wide transcriptome analysis indicated that Tie2-expressing FAPs promote blood vessel formation and muscle stem cell activation, while Vcam1-expressing sub-FAPs promote fibrosis.
"Importantly, this analysis revealed an association between these functional states and the inflammatory response of regenerating muscles," says Puri. "We observed that during acute injury, the inflammatory infiltrate—specifically macrophages—promptly cleared Vcam1-expressing sub-FAPs. This restricts their pro-fibrotic activity to transient collagen deposition, which favors optimal muscle stem cell division. However, in experimental conditions of macrophage depletion or in DMD muscles, in which macrophage activity is altered, an impaired clearance of Vcam1-expressing sub-FAPs resulted in chronic deposition of collagen and muscle fibrosis—one of the most deleterious events in DMD progression."
More information: Barbora Malecova et al, Dynamics of cellular states of fibro-adipogenic progenitors during myogenesis and muscular dystrophy, Nature Communications (2018). DOI: 10.1038/s41467-018-06068-6