Proof-of-concept HIV immunotherapy study passes Phase 1 safety trial
Preliminary results from a phase I clinical trial have demonstrated the safety and tolerability of a cell therapy involving the ex vivo expansion of T cells and their subsequent infusion into HIV-infected individuals previously treated with antiretroviral therapy (ART). The study appears September 21st in the journal Molecular Therapy.
"This study is focused on finding a way to re-educate the body's immune system to better fight HIV infection," says co-senior study author David Margolis of the University of North Carolina (UNC) at Chapel Hill. "We found that this approach of re-educating the immune cells and reinfusing them was safe, which was the primary goal of the study. The data from this trial will continue to help us design improved immunotherapies against HIV."
A game changer for patients living with HIV, ART has turned what was once a death sentence into a chronically managed disease. But it is not a cure, and the virus continues to persist within a latent reservoir that remains hidden from the immune system. Approaches using pharmacological HIV-latency-reversal agents to induce latent virus to express viral protein could make this reservoir vulnerable to T cells. However, the existing HIV-specific immune response in ART-treated individuals is insufficient to clear persistent infection, even in the presence of latency-reversal agents that induce HIV expression.
One safe avenue for harnessing T cell responses to fight HIV is adoptive cellular therapy. This procedure involves collecting T cells from a patient, growing them in the laboratory to increase their numbers, and then giving them back to the patient to help the immune system fight disease. Earlier adoptive-T-cell-therapy approaches for HIV had limited efficacy as a result of multiple factors. Since these earlier attempts, the adoptive-T-cell-therapy field has made significant advances, largely in the oncology field, that could help to overcome some of the pitfalls encountered with earlier T-cell-therapy approaches for HIV. T cells generated by these sophisticated methods of expansion have been safe and well tolerated, as well as highly effective.
"Before we can combine this approach with treatments meant to bring HIV out from hiding so the improved immune response can clear it from the body, we need to first establish that this immunotherapy approach is safe on its own," says co-senior study author Catherine Bollard of the Children's National Health System. "We have long-standing experience treating patients with virus-specific T cells targeting latent viruses such as Epstein-Barr virus and cytomegalovirus. Therefore, we were extremely excited to work with the UNC team to adapt this virus-specific T-cell-therapy approach to the HIV setting."
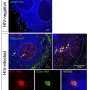
In the small proof-of-concept study, Margolis, Bollard, and their collaborators produced ex vivo expanded HIV-specific T cells (HXTCs); their long-term goal was to use HXTCs as part of a strategy to clear persistent HIV infection. The researchers administered two infusions of HXTCs over a 2 week period to six HIV-infected participants whose viral load had been reduced to an undetectable level by ART.
This treatment was well tolerated and had few adverse events. Moreover, two patients exhibited a detectable increase in T-cell-mediated antiviral activity after the two infusions, although the clinical significance of this mild-to-modest impact is unknown. When evaluating participants in aggregate, the research team found no overall enhancement of the magnitude of the HIV-specific immune response. This could be due to the low dose of the two infusions and the lack of strategies to promote the expansion of the T cells once they are in the body.
There was also no decrease in the size of the latent reservoir, most likely because of the absence of therapies such as latency-reversal agents, which are designed to perturb the reservoir and induce recognizable expression of HIV proteins that trigger immune responses. In the future, a critical question will be whether HXTC therapy in combination with latency-reversal agents can deplete the HIV reservoir to an extent that is measurable by current gold-standard assays of HIV latency. A study of HXTC in combination with the latency-reversal agent vorinostat is currently undergoing evaluation in an ongoing clinical trial.
"This is a promising advancement for the field," says first author Julia Sung of UNC, although she also cautions people against over-interpreting the results. "The study did not cure HIV and should not be interpreted as doing so, but we also are very encouraged by the safety data, so it should not be considered discouraging either. This paves the way for the next step, which is to combine this immunotherapy approach with latency-reversal therapy in order to wake up the HIV out of its latent state, where it is invisible to the immune system, then clear it out with the immunotherapy."
More information: Molecular Therapy, Sung et al.: "HIV-specific, Ex-Vivo Expanded T-Cell Therapy (HXTC): Feasibility, Safety, and Efficacy in ART-suppressed, HIV-infected Individuals" www.cell.com/molecular-therapy … 1525-0016(18)30400-3 , DOI: 10.1016/j.ymthe.2018.08.015