Clues to lupus's autoimmune origins in precursor cells
In the autoimmune disease systemic lupus erythematosus or SLE, the immune system produces antibodies against parts of the body itself. How cells that produce those antibodies escape the normal "checks and balances" has been unclear, but recent research from Emory University School of Medicine sheds light on a missing link.
Investigators led by Ignacio Sanz, MD, studied blood samples from 90 people living with SLE, focusing on a particular type of B cells. These "DN2" B cells are relatively scarce in healthy people but substantially increased in people with SLE.
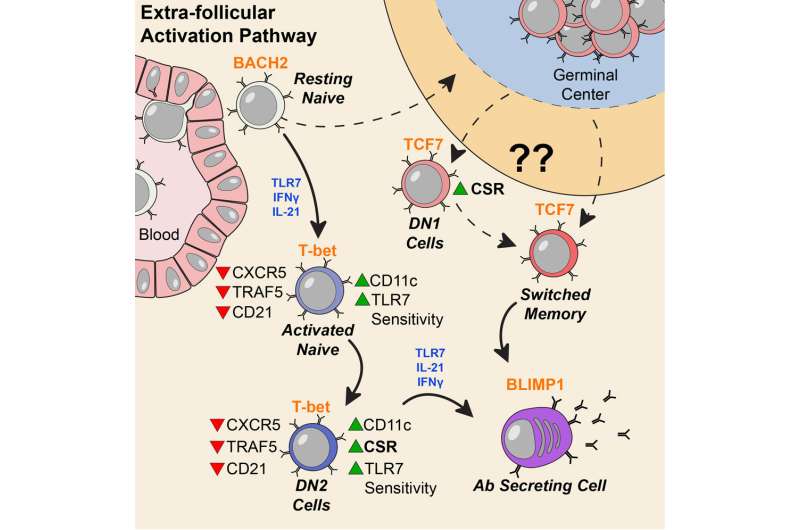
People with lupus can experience a variety of symptoms, such as fatigue, joint pain, skin rashes and kidney problems. Levels of the DN2 cells were higher in people with more severe disease or kidney problems. DN2 B cells are thought to be "extra-follicular," which means they are outside the B cell follicles, regions of the lymph nodes where B cells are activated in an immune response.
"Overall, our model is that a lot of lupus auto-antibodies come from a continuous churning out of new responses," says postdoctoral fellow Scott Jenks, Ph.D., co-first author of the paper. "There is good evidence that DN2 cells are part of the early B cell activation pathway happening outside B cells' normal homes in lymph nodes."
Previous research at Emory has shown that African American women have significantly higher rates of lupus than white women. In the current study, the researchers observed that the frequency of DN2 cells was greater in African American patients. Participants in the study were recruited by Emory, University of Rochester and Johns Hopkins.
Sanz, a Georgia Research Alliance Eminent Scholar, is director of the Lowance Center for Human Immunology and head of the division of rheumatology in the Department of Medicine at Emory University School of Medicine. Co-first authors are Jenks, postdoctoral fellow Kevin Cashman, Ph.D. and Esther Zumaquero-Martinez, Ph.D. at the University of Alabama. The lab of Frances Lund, Ph.D. at University of Alabama, as well as postdoc Urko Marigorta, Ph.D. from Georgia Tech contributed to the paper.
The researchers examined the characteristics of DN2 cells and the patterns of genes turned on in those cells. DN2 cells appear to be precursors to the plasmablasts that produce autoreactive antibodies, which cause so much trouble for people with lupus.
Plasmablasts are an important source of antibodies that help get rid of bacteria or viruses during an infection. But in lupus, subsets of B cells and plasmablasts persist in unhealthy ways. In general, we can think of plasmablasts as weapon factories, and B cells as a library of blueprints for various antibodies/weapons. Understanding where the problem plasmablasts come from can provide clues on how to target them and how to control the disease.
Molecular probing showed that DN2 cells in SLE patients are hypersensitive to activation through TLR7, a pathway by which the immune system senses viral infections. This may be how they get over-expanded, Jenks says.
"Our work provides further support for the importance for TLR7 in lupus pathology," he says. "Targeting TLR7 might both block the generation of pathological B cells and prevent their subsequent activation and differentiation into plasma cells."
Previous work in Sanz's lab had shown that a group of "activated naïve" B cells are precursors to the problem plasmablasts. Those cells are very similar, in their molecular markers, to DN2 cells. Jenks says the researchers are now figuring out the relationship between DN2 and activated naïve cells, as well as investigating additional intervention strategies that could specifically control those cells.
More information: Scott A. Jenks et al, Distinct Effector B Cells Induced by Unregulated Toll-like Receptor 7 Contribute to Pathogenic Responses in Systemic Lupus Erythematosus, Immunity (2018). DOI: 10.1016/j.immuni.2018.08.015