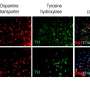
Intervening in glial cells protects neurons in Parkinson's model
Loss of dopaminergic neurons is a hallmark of Parkinson's disease pathology. When dopaminergic neurons are stressed, they send out a call for help to nearby glial cells that are tasked with providing neuronal support, protection and nourishment. Under particular molecular conditions, those calls for help can over-activate the glial cells, resulting in a cascade of inflammatory signaling that eventually contributes to the degradation of these neurons over time. Working in two fruit fly models of Parkinson's disease, researchers at the Buck Institute have characterized a novel molecular mechanism that orchestrates such a harmful cascade of inflammatory signaling and demonstrated that its disruption protects neurons as they age. The research, published in Cell Reports, provides a new framework for understanding the pathology of Parkinson's disease and offers an alternative approach for developing preventative treatments for a neurodegenerative disorder that afflicts millions of patients worldwide.
"We have known for some time that different forms of genetic or environmental stress in neurons can trigger a response in glial cells; now we've been able to identify a molecular mechanism that explains how neuronal stress can lead to activation of inflammatory signals in glial cells," said Buck professor Pejmun Haghighi, Ph.D., senior author of the study. "Working in flies allowed us to identify a vicious cycle: stressed neurons signal to the glia and trigger inflammatory signals in the glia, which become harmful for the neuron as the brain ages. Interestingly, the genetic components of this crosstalk are conserved between flies and humans, boosting our enthusiasm and confidence that future work might lead to novel therapeutic paradigms."
To induce Parkinson's-like neuronal defects, multiple sets of experiments were performed on flies that were genetically engineered to carry Parkinson's disease-related human genes or others that were exposed to a pesticide known as paraquat. In both cases, researchers identified Furin 1, a catalytic protein, in dopaminergic neurons as the initiator of an inflammatory signaling cascade in glial cells. Blocking this inflammatory signaling in the glial cells in both models of the disease reduced the toxic cross-talk and ultimately protected the neurons from degeneration.
"Furin 1 is the real culprit in the interaction between the neurons and glial cells. It's the 'finger' that pushes the switch on the signaling cascade," said postdoctoral fellow Elie Maksoud, Ph.D., the lead scientist on the study. "Furin 1 is a druggable target. Our hope is that treatments can be developed to reduce this toxic crosstalk before it becomes a serious problem for the dopaminergic neurons."
"We're looking at a new way to prevent Parkinson's, especially in those who have risk factors for the disease," said Haghighi. "The effects of this toxic signaling are age-dependent, they accumulate over time. The goal is to intervene as early in the disease process as possible." The researchers plan to use human cell culture models to further test the validity of the interactions.
More information: Elie Maksoud et al, A Neuron-Glial Trans-Signaling Cascade Mediates LRRK2-Induced Neurodegeneration, Cell Reports (2019). DOI: 10.1016/j.celrep.2019.01.077