Contraceptive drug shows promise for preventing and regressing cervical cancer
A new study in The American Journal of Pathology reports that medroxyprogesterone acetate (MPA), the active ingredient in the common contraceptive injection Depo-Provera, was effective in preventing the development of cervical cancer in mice with precancerous lesions. The drug also decreased existing precancerous lesions. If proven effective clinically, MPA may be a boon to women who do not have access to human papillomavirus (HPV) vaccines.
"Although HPV vaccines have been available since 2006, the incidence of precancerous lesions (cervical intraepithelial neoplasia or CIN) and cervical cancer due to HPV has not decreased substantially. The high cost and lack of a global vaccination program have limited the use of these vaccines. A non-invasive, efficient means to treat CIN is urgently needed," explained lead investigator Sang-Hyuk Chung, Ph.D., of the Center for Nuclear Receptors and Cell Signaling, Department of Biology and Biochemistry at the University of Houston, Houston, TX, USA.
Similar to cervical cancer progression in women, mouse cervical neoplastic disease develops through multiple stages, starting from CIN and culminating in invasive cancer.
Previously, efforts to develop a non-invasive treatment for CIN have been limited. Dr. Chung and co-investigators at the University of Houston had previously shown that MPA regresses cervical cancer in a mouse model expressing HPV genes responsible for cancer. In this study, they treated CIN-bearing mice with MPA.
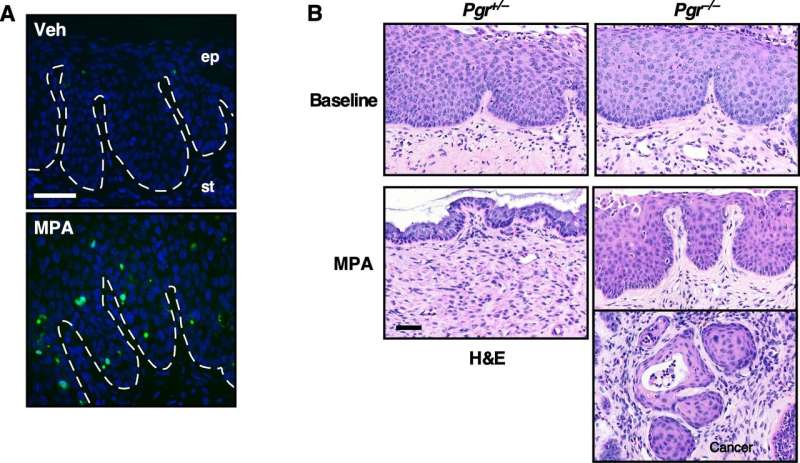
Investigators found that cervical cancer did not develop in mice receiving MPA. Further, CIN was absent in most MPA-treated mice, indicating that MPA may be "chemoprotective," not only preventing CIN from progressing to invasive cancer, but also promoting its regression.
The study determined that MPA inhibited cell proliferation and promoted apoptosis (cell death) in CIN lesions. In addition, the preventive effect of MPA was absent in HPV transgenic mice in which the expression of progesterone receptor (PR) was genetically prevented. These results suggest that MPA is efficient for treating PR-positive CIN lesions. PR-positivity may be a useful biomarker for selecting patients who may benefit from MPA in future clinical trials.
"We are optimistic because the mouse model we used in this study has been validated and revealed important mechanisms of cervical cancer," stated Dr. Chung. "MPA injectable suspensions are cheap and stable at room temperature; therefore, there is no need for special storage, which facilitates easy distribution. It is already used as the self-injectable contraceptive Depo-Provera, and thus translation into clinical use would be faster. MPA would be an effective chemoprevention agent for cervical cancer, particularly in women who do not have access to HPV vaccines. However, we should note that several studies have shown that MPA increases the risk of breast cancer."
Cervical cancer is the third most common and third most deadly cancer in women worldwide. HPV is considered a major factor in the development of cervical precancerous lesions and cancers. Although HPV vaccines are effective at preventing HPV infections, they may not be readily available to women in under-developed countries and those of low socio-economic status in developed countries. Surgical removal of a CIN can be clinically beneficial but has adverse effects including shortening the cervix and increasing the risk of complications in future pregnancies—undesirable outcomes in women of child-bearing age. CIN has negative impact on psychological and psychosocial wellness of women at levels similar to those caused by cervical cancer, underscoring the significance of this finding.
More information: American Journal of Pathology (2019). DOI: 10.1016/j.ajpath.2019.08.013