A common insulin signaling pathway across cancer and diabetes
An oncology researcher has made an unexpected contribution to the understanding of type 2 diabetes. In results published in Science Advances, Patrick Hu, M.D., associate professor of medicine at Vanderbilt University Medical Center, found a protein that modulates a signaling pathway often targeted by cancer therapies is also required for insulin biogenesis.
Hu and colleagues showed a protein controlling the PI3K/Akt pathway, a pathway targeted by more than 40 anti-tumor drugs, is absolutely required for the synthesis, processing and secretion of insulin. Hu initiated the discovery using the nematode worm Caenorhabditis elegans, a model system commonly used in research on genetics and development.
"We approached our work on the PI3K/Akt pathway from a cancer perspective, but this is a primordial pathway." Hu said. "It is equally relevant to diabetes and cancer. Understanding how this pathway is regulated could lead to new strategies to treat both diseases."
Insulin signaling is certainly involved in diabetes, but a related insulin-like growth factor signaling network is also implicated in cancer, Hu explained. In C. elegans, a primordial pathway exists that likely gave rise to both human pathways, providing a convenient research model.
"Both networks involve PI3K/Akt in humans, and we were looking for new components of this pathway," Hu said.
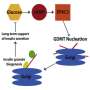
The researchers used a forward genetic approach to screen C. elegans worms for components of the pathway that are altered during abnormal insulin signaling. They landed on TRAP-alpha, a highly conserved protein across worms, flies and mammalian systems, including humans.
TRAP-alpha sits on a structure inside cells called the endoplasmic reticulum (ER), where it helps make proteins that will eventually be secreted. Deleting the worm equivalent of TRAP-alpha activated ER stress responses, the researchers found.
Given some people with type 2 diabetes have common genetic variants in the TRAP-alpha gene, Hu moved the experiments to pancreatic beta cells.
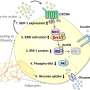
Hu collaborated with Ming Liu, M.D. and Peter Arvan, M.D. of the University of Michigan to delete TRAP-alpha from rat beta cells. The deletion caused a 90 percent reduction in total insulin content inside the cells. Instead of being shuttled through the ER for conversion to insulin and secretion, most parent molecules of insulin were degraded, and those that escaped degradation accumulated inside beta cells. They were never processed to insulin, or secreted. The finding shows how without TRAP-alpha, insulin biogenesis is drastically impaired.
Said Hu, "TRAP-alpha is the first situation where we've identified a mutant in the worm and then were able to move it into mammalian cell culture to show it affects a disease phenotype."
In both models, the researchers found deleting TRAP-alpha triggered the ER unfolded protein response. The cells detected unfolded proteins accumulating inside them, and decreased corresponding gene expression to combat it. The cells also increased expression of chaperone proteins that help fold proteins properly.
"We're moving toward the role of TRAP-alpha in maintaining protein homeostasis," Hu said. "Maintaining proper protein folding in the ER is certainly important for cellular health, and it likely contributes to human health in general."
Beyond diabetes, many other diseases are associated with abnormal protein folding responses and protein imbalances. These include neurodegenerative diseases such as amyotrophic lateral sclerosis and Alzheimer's disease.
"It's likely other secreted molecules besides insulin might be affected by TRAP-alpha deletion," Hu said. "If we can understand the broader role that TRAP-alpha is playing in maintaining protein homeostasis, we might develop new ways to approach other diseases, too."
More information: "Requirement for translocon-associated protein (TRAP) α in insulin biogenesis" Science Advances (2019). advances.sciencemag.org/content/5/12/eaax0292