Hope for patients with a rare genetic condition linked to severe infections
A team of researchers at CHU Sainte-Justine and Université de Montréal has shed light on the mechanisms that underlie a rare genetic condition by creating the first cellular model of the disease. The study's findings were published today in the Journal of Allergy and Clinical Immunology.
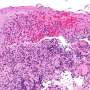
Chronic granulomatous disease (CGD) is a rare hereditary condition that affects one in every 217,000 people worldwide and typically strikes patients at an early age.
"It is a primary innate immune defect that typically leads to severe, recurrent infections caused by bacteria and fungi, as well as potentially disabling lung inflammation or inflammatory colitis similar to Crohn's disease," said senior author Dr. Fabien Touzot, a clinical assistant professor in pediatric medicine at UdeM and researcher in pediatric immunology and hematology at CHU Sainte-Justine.
"Currently, patients are forced to take antibiotics and anti-inflammatory drugs for the rest of their lives."
Gene editing shows the way forward
To better understand the mechanisms that trigger inflammation in patients with CGD, Touzot and his research team created the very first cellular model of the disease in their labs at CHU Sainte-Justine. They then used a technique known as gene editing to recreate and introduce into their model a genetic mutation that causes the disease. This allowed them to model the inflammatory response observed in patients and to study its mechanisms.
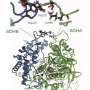
"CGD is a hereditary illness caused by mutations in the NADPH oxidase enzyme. These mutations prevent white blood cells from working properly and, as a result, the patient's body can no longer defend itself against certain kinds of bacteria and fungi," said researcher Aissa Benyoucef, the study's first author.
"More than 90% of affected patients have inflammation that appears to be unrelated to infectious agents," he added. "Treating this inflammation is difficult, since it can put patients at increased risk of infection, which can sometimes be fatal. A better understanding of the mechanisms underlying the disease could help us develop new and more effective treatment strategies."
The research team showed that restoring NADPH oxydase function in defective cells would put the immune process back on track, thereby proving that this genetic mutation plays a direct role in causing inflammation.
"CHU Sainte-Justine is one of Quebec's leading centres of expertise in rare genetic diseases," said Touzot. "We're proud to serve patients by expanding the knowledge base in this area and by contributing to the development of precision medicine."
The new cellular model will be useful for the development of targeted treatments that are less toxic and more effective in treating inflammation, significantly improving patient quality of life, according to the researchers.
More information: Aissa Benyoucef et al, CRISPR gene-engineered CYBBko THP-1 cell lines highlight the crucial role of NADPH-induced reactive oxygen species for regulating inflammasome activation, Journal of Allergy and Clinical Immunology (2020). DOI: 10.1016/j.jaci.2019.12.913