New antibody maturation technique yields high-affinity Arginase 2 antibody
Researchers at the Cancer Research UK-AstraZeneca Antibody Alliance Laboratory in Cambridge, UK, have pioneered the development of an innovative affinity maturation technique to generate an inhibitory, high-affinity antibody against Arginase 2 (ARG2), an enzyme implicated in major human diseases including cancer. Their findings are published in mAbs today (Thursday), following publication of the method in Proceedings of the National Academy of Sciences.
ARG2 targets and destroys L-arginine, an amino acid critical for immune cells to fight diseases such as cancer, as well as infections. Although ARG2 is necessary to control arginine levels in the body, it is overexpressed in various types of cancer, including pancreatic ductal adenocarcinoma, bowel cancer and acute myeloid leukemia.
In these cancers, the excess ARG2 present decimates the levels of L-arginine around tumors and hinders the immune cells that need it to function, creating an immunosuppressive local environment that allows tumors to grow unchecked, 'hidden' from the immune system. ARG2 is therefore an attractive target for inhibitory therapeutic antibodies that could help restore immune function against cancer cells.
Dr. Maria Groves and her team at the Cancer Research UK-AstraZeneca Antibody Alliance Laboratory in Cambridge, in collaboration with the late Professor Cerundolo and his team at Oxford University, and Professor Mark Carr's structural biology group at the University of Leicester, have generated a highly promising potential therapeutic antibody against ARG2. The lead antibody, called C0021158, is the first product of the researchers' innovative antibody affinity maturation approach reported last month in PNAS and boasts a full complement of desirable properties, including high affinity binding and complete inhibition of ARG2 activity.
Traditional affinity maturation approaches have focused on exploring sequence diversity in only a few specific regions of antibodies, limiting the improvements that could be obtained in candidate therapeutics—particularly for antibodies that are challenging to mature. The innovative approach used to develop C0021158 allowed the researchers to identify antibodies with non-predictable combinations of amino acid sequence changes that generated a significantly-improved affinity and potency of inhibition against ARG2. The pioneering anti-ARG2 therapeutic antibody work dramatically illustrates the advantages of the new unbiased approach.
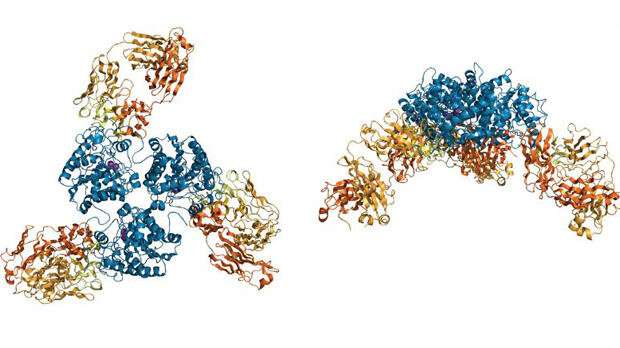
Characterisation of C0021158 highlighted the resounding success of the researchers in generating a therapeutic candidate, which demonstrates complete and highly potent inhibition of ARG2 activity; in laboratory assays, the antibody restored the proliferation of immune cells that were suppressed by ARG2-driven arginine deficiency. Protein crystallography performed at the University of Leicester provided key structural and molecular insights into the mechanism of antibody-mediated inhibition, revealing dramatic changes in antibody interaction with ARG2 resulting from affinity maturation. The inhibitory antibody binding induced dramatic changes in the structure of ARG2, resulting in conformation changes at the active site of the enzyme that prevent both ARG2 activity and productive binding of its arginine target.
The researchers expect that the techniques described in these papers will launch the discovery of a new generation of high-affinity and high-potency therapeutics made possible by unbiased affinity maturation. This will be of particular importance in circumstances where candidate antibodies are difficult to mature, and will have treatment applications in cancer and beyond.
Study author Dr. Maria Groves, from the Cancer Research UK-AstraZeneca Antibody Alliance Laboratory, said: "It takes a tremendous amount of time and resources to bring a new therapeutic antibody to the clinic, so we need them to be the best they can be. This first success gives me confidence that our unbiased libraries will produce stronger, more efficient antibodies, and support the delivery of novel oncology therapeutics in the future."
There's still a long way to go with the new ARG2 antibody, and the researchers are now hoping to partner with a company to help develop the antibody and progress it into the clinic for oncology and non-oncology indications. For oncology, they believe that it may help target tumors that have been hiding from the immune system, when used in combination with chemotherapy or immunotherapy.
The structural biology lead for the project, Professor Mark Carr, from the Institute of Structural and Chemical Biology at the University of Leicester said: "The outstanding success of the ARG2 therapeutic antibody project is an exemplar of the substantial benefits to be gained in drug discovery from harnessing the complementary scientific expertise and knowledge available in the pharmaceutical industry, biomedical charity and university sectors of the UK life sciences community. The outcomes from this project point to a successful future for the joint CRUK-AstraZeneca Antibody Alliance Laboratory and my structural biology group look forward to further successful collaborations."
Dr. Andreas Hadjinicolaou, from the MRC Human Immunology Unit at the University of Oxford, said: "Our collaboration with the Antibody Alliance Laboratory has transformed an idea born in Oxford University to successful fruition in an extremely efficient and scientifically robust manner. The lab applied innovative antibody technology to stop the immune evasion effects of the ARG2 enzyme in cancer. This focused collaboration generated exceptional outcomes and the output from the lab will undoubtedly serve to deliver novel oncology therapeutics for the future. It has been a pleasure and a privilege to be part of this effort."
More information: Mark Austin et al. Structural and functional characterization of C0021158, a high-affinity monoclonal antibody that inhibits Arginase 2 function via a novel non-competitive mechanism of action, mAbs (2020). DOI: 10.1080/19420862.2020.1801230
Denice T. Y. Chan et al. Extensive sequence and structural evolution of Arginase 2 inhibitory antibodies enabled by an unbiased approach to affinity maturation, Proceedings of the National Academy of Sciences (2020). DOI: 10.1073/pnas.1919565117
Tomasz M. Grzywa et al. Myeloid Cell-Derived Arginase in Cancer Immune Response, Frontiers in Immunology (2020). DOI: 10.3389/fimmu.2020.00938