Researchers develop new method to print tiny, functional organs
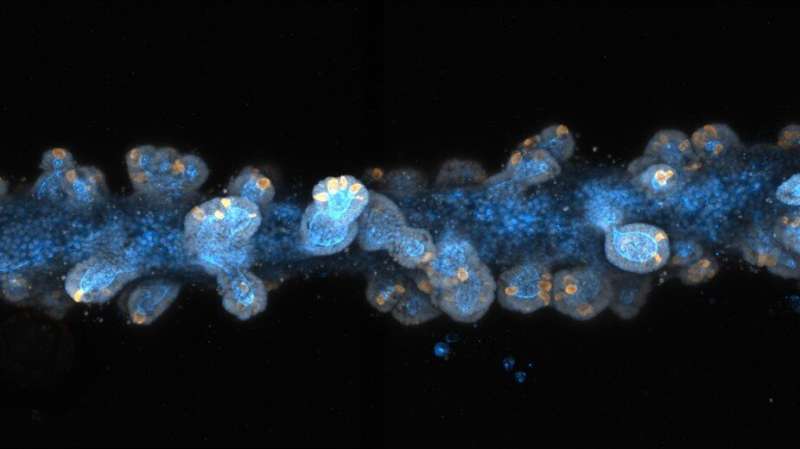
Researchers at EPFL have developed an approach to print tiny tissues that look and function almost like their full-sized counterpart. Measuring just a few centimeters across, the mini-tissues could allow scientists to study biological processes—and even test new treatment approaches—in ways that were previously not possible.
For years, mini versions of organs such as the brain, kidney and lung—known as "organoids"—have been grown from stem cells. Organoids promise to cut down on the need for animal testing and offer better models to study how human organs form and how that process goes awry in disease. However, conventional approaches to grow organoids result in stem cells assembling into micro- to millimeter-sized, hollow spheres. "That is non-physiological, because many organs, such as the intestine or the airway, are tube-shaped and much larger," says Matthias Lütolf, a professor at EPFL's Institute of Bioengineering, who led the study published today in Nature Materials.
To develop larger organoids that resemble their normal counterparts, Lütolf and his team turned to bioprinting. Just as 3-D-printers allow people to create everyday objects, similar technology can help bioengineers to assemble living tissues. But instead of the plastics or powders used in conventional 3-D-printers, bioprinters use bioinks—liquids or gels that encapsulate living cells. "Bioprinting is very compelling because it allows you to deposit cells anywhere in 3-D space, so you could think of arranging cells into an organ-like configuration such as a tube," Lütolf says.
The researchers designed a customized bioprinting set-up consisting of a microscope and a device that can aspirate and deposit cells through a thin nozzle coupled to a syringe pump. On the microscope stage, the team installed a plate containing a gel that resembles the complex extracellular environment found in many tissues. This type of gel, Lütolf notes, "is incredibly potent to allow cells to form a tissue, but because it's difficult to handle people haven't really exploited it for bioprinting."
By moving the microscope stage and monitoring constantly the process through the microscope lens, the researchers were able to deposit into the gel a line of intestinal stem cells that measured a few centimeters in length. "The cool thing about using a microscope is that you can always see what you're doing and you can watch what cells do—you're not blind," Lütolf says. "In other bioprinting approaches, you don't see what's going on."
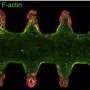
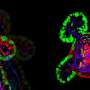
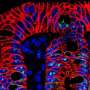
Once stem cells were seeded, Lütolf says, "magic happened": the cells started to grow and interact with each other, forming a continuous, tube-shaped tissue that mimicked many of the anatomical and functional features of a regular gut. The lab-grown guts, which reached up to three centimeters in size, were composed of crypt-shaped pockets with stem cells and they contained the same specialized absorptive and secretory cells as those found in a full-sized intestine. The mini-guts' secretory cells were also able to secrete antimicrobial molecules in response to specific stimuli.
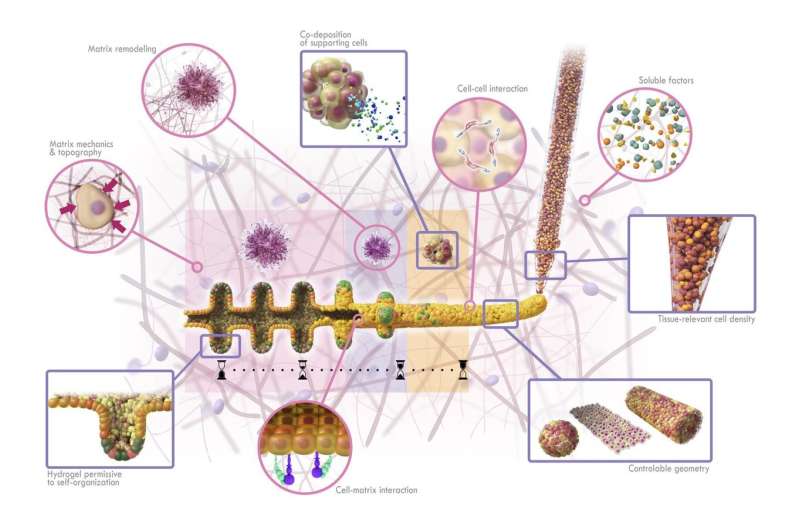
What makes the newly developed approach different—and more successful—than other methods to grow organoids is that it combines the flexibility and precision of 3-D-printing with the ability of stem cells to grow and organize themselves. "We allow the biology to happen—that's absolutely crucial," Lütolf says.
By bioprinting organoid-forming cells from the gastrointestinal tract, the researchers also generated portions of the stomach, small intestine and colon, which then formed interconnected mini versions of their parent organs. With traditional methods for growing organoids, Lütolf notes, "you can grow either stomach organoids or intestinal organoids—whereas with bioprinting, you can combine different cell types and arrange them in different ways," he says.
While the mini-organs perform the functions of their normal counterparts, their use in regenerative medicine, including for replacing human tissues and organs, is still years away, Lütolf says. But he notes that the newly developed approach could be used to build tissue models for human diseases, including cancer, and for testing how drug candidates act on specific cell types within a tissue.
Lütolf's team now hopes to use bioprinting to build airway tubes to study viral infection, for example with the SARS-CoV-2 virus that causes COVID-19. The infected mini-airways could then help to test different treatment approaches. "A big advantage is that, with a microscope, you can watch as infection evolves, so you can quantify and study what happens," Lütolf says. "That's an exciting perspective."
More information: Jonathan A. Brassard et al. Recapitulating macro-scale tissue self-organization through organoid bioprinting, Nature Materials (2020). DOI: 10.1038/s41563-020-00803-5