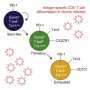
Clarifying the problem of T-cell 'exhaustion'
Researchers in the Perelman School of Medicine at the University of Pennsylvania have illuminated an important limitation of the immune system in prolonged battles against cancers or viruses: T cells, which are among the most powerful weapons in the immune systems of humans and other vertebrates, remain substantially programmed to stay exhausted even many weeks after exposure to a virus ended. The findings were published today in Nature Immunology.
Scientists have known that T cells can lose their ability to fight viruses and tumors when they have prolonged exposure to these enemies. They have hoped that this "T cell exhaustion" phenomenon could be reversed relatively easily, for example when the T cells are no longer exposed to the virus or tumor in question. Scientists now will need to take into account this limitation, including when devising immune-based therapies against chronic viral infections and cancers.
"Our findings suggest that once T cells become exhausted, they remain fundamentally 'wired' to be exhausted—thus it may be hard to get them to become effective virus- and cancer-fighters again," said study senior author E. John Wherry, Ph.D., chair of the department of Systems Pharmacology and Translational Therapeutics and director of the Penn Institute of Immunology in the Perelman School of Medicine at the University of Pennsylvania.
The recognition of the T cell exhaustion problem emerged about two decades ago from studies of long-term viral infections, including studies by Wherry and his laboratory. Scientists eventually concluded that long-term exposure not only to viruses but also to cancerous tumors could exhaust T cells. Exhausted T cells start producing much lower amounts of immune response-stimulating proteins, and generally become less able to kill virus-infected cells or tumor cells.
Researchers have hoped that by blocking certain activity-inhibiting receptors on exhausted T cells, they could reinvigorate the T cells in cancer patients and in patients infected long-term with viruses such as HIV or Hepatitis C virus. However, there is evidence now that such reinvigoration, for example with cancer drugs called PD-1 inhibitors, tends to be incomplete and temporary.
One question that hasn't been resolved is whether, and to what extent, exhausted T cells can recover normal functions if they no longer have exposure to the virus or tumor that exhausted them. In other words, if a person goes into long-term remission from cancer, will his or her anti-tumor T cells become un-exhausted, providing some measure of immunity against future cancer recurrence?
Wherry and colleagues, including first author Mohamed Abdel-Hakeem, Ph.D., a postdoctoral research associate in the Wherry Laboratory, addressed this question in the new study. They examined mouse T cells that had been exhausted by chronic exposure to a mouse virus called LCMV—long used as a standard model of T cell-exhausting infection.
The researchers found that most of the exhausted T cells died when they were no longer exposed to LCMV. The small proportion that survived recovered some of the gene expression patterns that would be expected in normal, memory-type T cells that help sustain a long-term immune defense following infection. But for the most part, the T cells remained programmed to stay in the exhausted, ineffective state, especially when called into action again upon reinfection.
A cell is programmed to be in a certain state or identity by an 'epigenetic' system of molecules that control which genes are active or inactive in the cell. These molecules often work by altering the structure of coiled DNA in the nucleus of the cell, making some genes more accessible to gene-copying enzymes, and others less accessible. In the study, the researchers observed that the epigenetic, DNA-structure changes that are characteristic of exhausted T cells mostly remained stable in these cells after LCMV exposure ended.
"Exhaustion apparently leaves durable 'epigenetic scars' in T cells that constrain their ability to support an immune response. These findings point to a need to discover how to reverse that epigenetic scarring," Wherry said.
In principle, future treatments that reverse those epigenetic changes could turn exhausted T cells in patients into normal memory T cells again. Epigenetic drugs have long been a hope of cancer therapy because tumor cells also undergo epigenetic dysregulation. These drugs, however, remain somewhat unwieldly or imprecise in their effects. However, if the right epigenetic drugs can be found to modulate immune cells that could help patients fight ongoing cancers or viral infections, and could also provide patients with stronger long-term immunity following the eradication of tumors or chronic viral infections. Studies at Penn Medicine are ongoing to manipulate the epigenetics of exhausted T cells in combination with immunotherapy.
More information: Mohamed S. Abdel-Hakeem et al, Epigenetic scarring of exhausted T cells hinders memory differentiation upon eliminating chronic antigenic stimulation, Nature Immunology (2021). DOI: 10.1038/s41590-021-00975-5