HMGB1 released from nociceptors mediates inflammation
In an effort to better understand inflammation within the body, researchers at The Feinstein Institutes for Medical Research—the global scientific home of bioelectronic medicine—successfully controlled the neurons that release molecular proteins and turn on/off inflammation. The preclinical research recently published in the journal Proceedings of the National Academy of Sciences (PNAS). The study points to a new approach to treat diseases such as arthritis, which are characterized by inflammation and pain.
Inflammation is the body's defense response to injury and infection triggered by molecular proteins, such as high mobility group box 1 protein (HMGB1). These molecules also stimulate sensory neurons, termed nociceptors. Activation of nociceptors controls the inflammation through release of neuropeptides producing neuroinflammation. While important, if unresolved inflammation can be harmful, resulting in autoimmune or autoinflammatory disorders like rheumatoid arthritis or Crohn's disease.
"Using light or genetic tools, we can turn off and on the switch that controls inflammation in the body," said Sangeeta S. Chavan, Ph.D., professor in the Institute of Bioelectronic Medicine at the Feinstein Institutes. "This preclinical bioelectronic medicine research is paving the way for a variety of new treatment options for people living with serious, chronic health conditions."
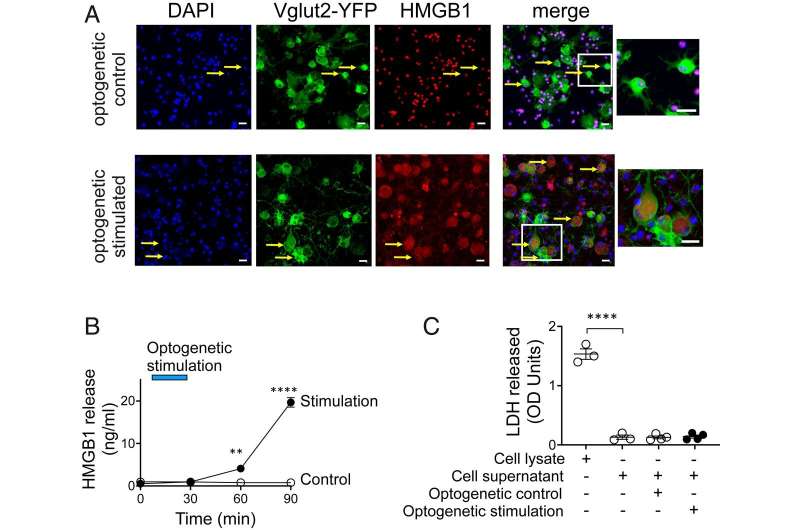
The study, led by Dr. Chavan and associate professor Huan Yang, Ph.D., combines optogenetics, neuronal-specific deletion (or removal) and preclinical models of inflammatory diseases while monitoring inflammation and neuropathic pain. The results show that nociceptor HMGB1 is required for a neuroinflammatory response to injury. These experiments provide direct evidence that nociceptor-related pain can be prevented by targeting HMGB1—a potential therapy of neuroinflammatory diseases.
"Our new methodology allows us to develop ways to regulate inflammation mediated by neurons," said Dr. Yang. "The optogenetic and neuronal-specific ablation strategies show us the critical role HMGB1 plays in neuroinflammation and pain."
The results show that a new paradigm for nervous system integration of environmental signals to stimulate inflammation is fundamental to spark an immune system defense during infection and injury. The research suggests that targeting HMGB1 may constitute a new therapeutic approach for treating a variety of diseases.
"After 30 years of researchers studying how to turn off the immune system, we recently sought a way to turn it on," said Kevin J. Tracey, MD, president and CEO of the Feinstein Institutes. "Thanks to this pivot in research, Drs. Yang and Chavan discovered that nerves release a molecule to produce inflammation in the body, which could lead to a new strategy to develop pharmaceutical and bioelectronic therapies."
Dr. Chavan and the Institute of Bioelectronic Medicine continue to advance this emerging field of science which combines molecular medicine, neuroscience and bioengineering to study how to use devices to treat diseases and injury. Dr. Chavan reported in a 2020 study also published in PNAS, the discovery of a small cluster of neurons within the brain that is responsible for controlling the body's immune response and the release of cytokines, which leads to inflammation in the body.
More information: Huan Yang et al, HMGB1 released from nociceptors mediates inflammation, Proceedings of the National Academy of Sciences (2021). DOI: 10.1073/pnas.2102034118