Mixed vaccine schedules offer strong booster responses after two doses of CoronaVac vaccine
A third booster dose of either the ChAdOx1 nCoV-19 (Oxford-AstraZeneca), BNT162b2 (Pfizer-BioNTech), AD26.COV2-S (Janssen) or CoronaVac (SinoVac) coronavirus vaccine induce a significant increase in antibody levels in those who have previously received two doses of CoronaVac. The strongest responses were seen with mixed schedules, including against the delta and omicron variants of concern.
The results from a study funded by the Ministry of Health, Brazil, and conducted by researchers from Brazil and the University of Oxford have been published as a peer-reviewed paper in The Lancet.
Professor Sir Andrew Pollard, Director of the Oxford Vaccine Group and lead of the study, said:
"This study shows that the inactivated vaccine, CoronaVac, can be successfully boosted with a range of different vaccines, with the strongest responses when a viral vector or RNA vaccine is used. The global priority remains first and second doses but this study provides important options for policymakers in the many countries where inactivated vaccines, like CoronaVac, have been used."
Professor Sue Ann Costa Clemens CBE of the Oxford Vaccine Group, and lead of the study in Brazil, said:
"The new data presented here show the extraordinary response to a third dose of coronavirus vaccines in a heterologous vaccine schedule, supporting the recommendation of the Brazilian Ministry of Health to use RNA and viral vector vaccines for Brazil's booster program. These data will also guide other low- and middle-income countries in setting up the most optimal and affordable booster programs."
In the publication, the researchers detail the findings from a randomized study of 1,240 volunteers in São Paulo, and Salvador, Brazil, 1,205 of whom were eligible for inclusion in the final analysis.
These volunteers were divided into four groups, receiving a booster dose of either the Oxford-AstraZeneca, Pfizer-BioNTech, Janssen or CoronaVac coronavirus vaccines, six months after their prior immunisations with CoronaVac.
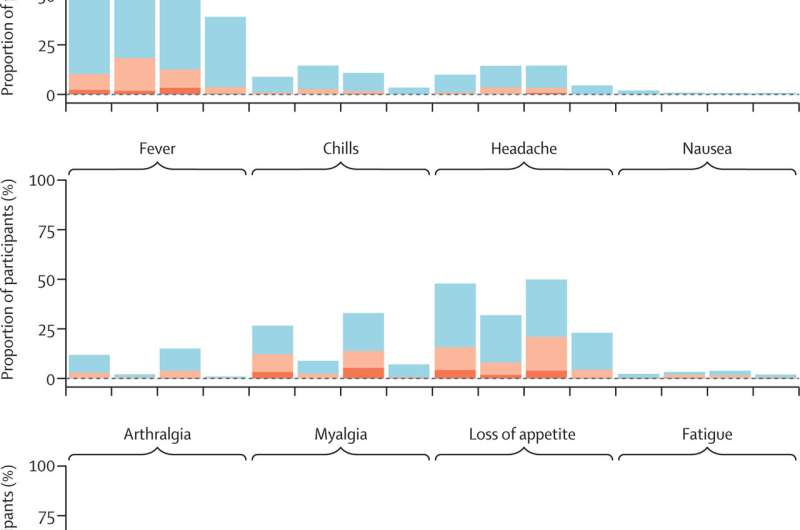
Antibody levels were low prior to delivery of the booster doses, with only 20.4% of adults aged 18–60 and 8.9% of adults aged over 60 having detectable levels of neutralizing antibodies. These were seen to significantly increase across every booster vaccine regimen, across all groups, 28 days post-vaccination—with the highest responses recorded after the RNA and viral vector vaccines, including responses against delta and omicron.
This increase in binding and neutralizing antibodies may improve protection against infection in vaccinated, and boosted, individuals, the researchers conclude.
More information: Sue Ann Costa Clemens et al, Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study, The Lancet (2022). DOI: 10.1016/S0140-6736(22)00094-0