Canada approves first domestic COVID-19 vaccine
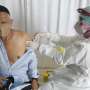
Canadian health authorities approved Thursday the first domestic COVID-19 vaccine, Quebec City-based biopharmaceutical firm Medicago and its partner GlaxoSmithKline announced.
This comes after more than 90 percent of Canadian adults have already received two jabs of other vaccines, but about half of the population has yet to get a booster shot.
Calling Health Canada's approval of its Covifenz vaccine "a significant milestone for Canada in the fight against the pandemic," Medicago chief executive Takashi Nagao said the company was already "manufacturing doses to start fulfilling its order."
Ottawa had pre-ordered up to 76 million doses of Covifenz.
Industry Minister Francois-Philippe Champagne said the development of the vaccine also marked a reversal of a decades-long decline in Canada's biomanufacturing capabilities, which the pandemic had exposed.
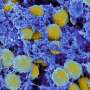
"This is the first authorized COVID-19 vaccine developed by a Canadian-based company, and the first that uses a plant-based protein technology," said a government statement.
Ottawa had previously approved six other COVID vaccines, including AstraZeneca, Moderna and Pfizer.
According to Health Canada, clinical trials have shown the two-dose Covifenz vaccine to be 71 percent effective in protecting adults aged 18 to 64 against coronavirus infections.
© 2022 AFP