Targeting TGF-β for treatment of osteogenesis imperfecta
A new study led by Baylor College of Medicine identifies an underlying mechanism of pathogenesis for osteogenesis imperfecta (OI) in human bone. The report, published in the Journal of Clinical Investigation, also shows the results of a Phase 1 clinical trial testing the safety and effectiveness of an antibody treatment targeting that pathway.
Osteogenesis imperfecta is the most common skeletal dysplasia and causes bone fragility in children and adults. There is no FDA-approved treatment for OI. Previous research conducted at Baylor has shown that a protein called transforming growth factor-β (TGF-β) is upregulated in bones and connective tissues of mouse models of common forms of OI, suggesting a common signaling pathway in OI and a potential therapeutic target.
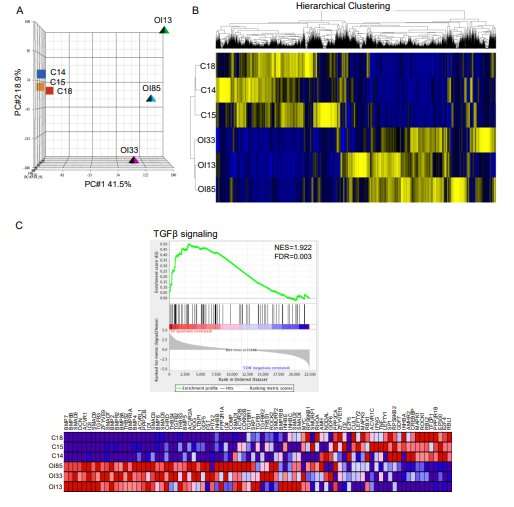
Through the Brittle Bone Disorders Consortium, a part of the NIH-funded Rare Diseases Clinical Research Network, Baylor researchers conducted a new study to prove that the results of their animal studies could be replicated in humans. They examined human bone samples from OI patients at consortium sites at Baylor/Texas Children's Hospital and the University of Nebraska Medical Center. A multiomic approach analyzing RNA and protein expression revealed that TGF-β was upregulated when compared to non-OI bone.
To translate these findings to the clinic, the Baylor team tested fresolimumab, a monoclonal antibody therapy that neutralizes TGF-β in a Phase 1 clinical trial conducted at BBDC sites at Baylor and Oregon Health & Science University. Eight adults with moderate to severe OI received a single infusion of fresolimumab provided by Sanofi.
"In patients with moderate forms of OI, we saw a significant increase in bone density at three and six months, which is a really dramatic effect after only a single infusion," said Dr. Brendan Lee, corresponding author of the study, professor and chair of the Department of Molecular and Human Genetics and Robert and Janice McNair Endowed Chair in Molecular and Human Genetics at Baylor. "This could be an effective therapy targeting the mechanisms underlying disease in OI."
Fresolimumab has been used before to treat other diseases, including cancer, and it can have significant side effects. But in this study, the drug was well tolerated with only minor side effects. Lee says targeting bone offers potential safety advantages.
"Human bone turns over every three to six months, which allows us to decrease the administration frequency as the therapeutic effect on bone lasts beyond the time the drug is in the body," said Lee, who also is principal investigator of the BBDC.
The data showed a stronger effect in patients with moderate OI, indicating that dosage may need to be adjusted based on severity of disease in future trials. Plans for additional trials are already underway, with Sanofi set to lead a larger Phase 1b clinical trial to test safety, tolerability and impact on bone density (NCT05231668).
According to Lee, this therapy could have additional benefits for OI patients.
"In OI patients, TGF-β is found at higher levels in connective tissues like the lung as well as bone," Lee said. "We hope future trials will determine whether fresolimumab can help with other OI symptoms affecting connective tissue."
More information: I-Wen Song et al, Targeting transforming growth factor- β (TGF-β) for treatment of osteogenesis imperfecta, Journal of Clinical Investigation (2022). DOI: 10.1172/JCI152571
Ingo Grafe et al, Excessive transforming growth factor-β signaling is a common mechanism in osteogenesis imperfecta, Nature Medicine (2014). DOI: 10.1038/nm.3544