Genome-wide intricacies of cancer inhibitor untangled
A newly discovered inhibitor of a common cancer-causing protein operates selectively, reducing expression of genes that fuel rampant cell proliferation and alter the epigenome, according to a Northwestern Medicine study published in Science Advances.
The protein, called MYC, was previously considered "undruggable," but these new findings demonstrate that certain inhibitors may selectively affect pro-cancer processes, according to Debabrata Chakravarti, Ph.D., the Anna Lapham Professor of Obstetrics and Gynecology, vice chair for translational research in the Department of Obstetrics and Gynecology and co-senior author of the study.
"These findings create the foundation to build and test all new lines of compounds for MYC inhibition," said Chakravarti, who is also a professor of Pharmacology and assistant director of shared resources at the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
MYC is a transcription factor protein that generally upregulates genes involved in cell proliferation. The transcription factor is persistently hyper-expressed in many cancers, but designing inhibitors is complicated by both the protein's unusual structure and its considerable influence beyond cell proliferation, meaning previous inhibitors had serious toxic side-effects.
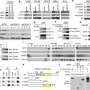
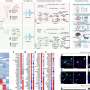
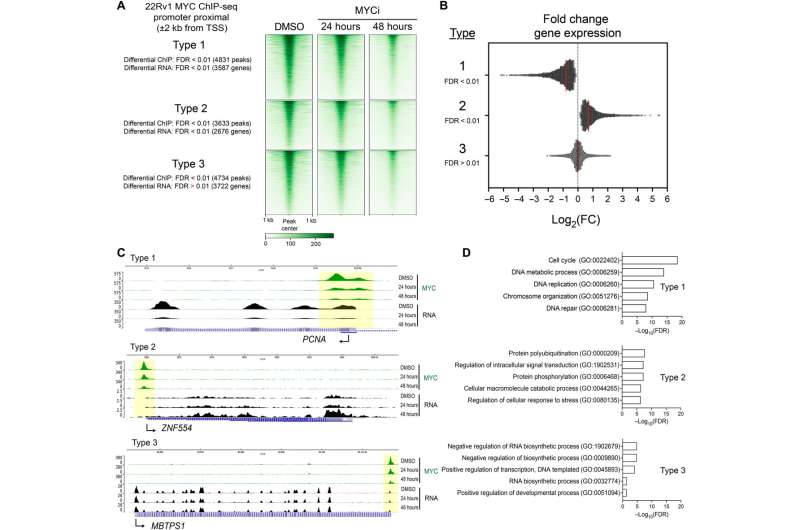
In the current study, investigators administered a small-molecule inhibitor called MYCi975 to prostate cancer cells, examining the effects on gene expression throughout the human genome. Rather than broad downregulation of all MYC targets, unexpectedly, the MYCi inhibitor had variable effects—upregulating some genes, downregulating others and sparing some altogether.
"This is how we could glean information about selectivity of the drug," said Austin Holmes, a recent graduate of the Driskill Graduate Program in Life Sciences (DGP) and the lead author of the study.
Importantly, some genes which are essential for cellular functionality—and that may have been responsible for toxicity in previous MYC inhibitors—were untouched, according to Sarki Abdulkadir, MD, Ph.D., the John T. Grayhack, MD, Professor of Urological Research, vice chair for research in the Department of Urology and co-senior author of the study.
"Not all MYC functions were abrogated," said Abdulkadir, who is also a professor of Pathology and a principal investigator of the Specialized Program of Research Excellence (SPORE) in prostate cancer at the Lurie Cancer Center. "This gives us some selectivity that has not been shown before."
On the other hand, several cancer-associated targets of MYC were downregulated, preserving the inhibitor's anti-cancer effects. The realization that MYC inhibitors can exert a variable effect on downstream gene targets of MYC provides a new path forward for drug development, according to Chakravarti.
"Excitingly, in animal models, MYCi975 enhances the efficacy of enzalutamide anti-hormone therapy. Our hope is to use this platform to analyze the second or third generation of compounds, and to explore how these drugs may interact with established anti-cancer therapies," Chakravarti said. "This study is also a prototype of how collaborative research can lead to significant discoveries in biomedical research."
More information: Austin G. Holmes et al, A MYC inhibitor selectively alters the MYC and MAX cistromes and modulates the epigenomic landscape to regulate target gene expression, Science Advances (2022). DOI: 10.1126/sciadv.abh3635