Aldose reductase promotes diet-induced obesity
High-fat diet (HFD) feeding in mice promotes induction of aldose reductase (AR) activity, expression, and senescence of adipocytes in subcutaneous adipose tissue (scAT), according to a new study in Obesity journal.
"Our data demonstrate that aldose reductase gene expression increases in scAT of obese humans and mice, and that an inhibitor of aldose reductase attenuates weight gain, reduces adipocyte senescence and promotes lipolysis in HFD-fed mice; these data pave the way for testing these inhibitors as therapeutic adjuncts in treating patients with obesity," said Ravichandran Ramasamy, Ph.D., Diabetes Research Program, Department of Medicine, NYU Grossman School of Medicine in New York. Ramasamy is the corresponding author of the study.
All mice studied were male, had free access to water and food, and were subjected to 12-hour light/dark cycles. AR knockout mice and wild-type littermates were used. All mice used in the studies were randomly assigned to treatment groups. As for diet, the eight-week-old mice were fed an HFD (60% of calories from lard) or standard chow (13% calories from fat) for 12-weeks and examined expression of the senescence marker. In specific studies, after 11 weeks of HFD feeding, the aldose reductase inhibitor (ARI) Zopolrestat (Zop), 2.5mg/kg body weight, or vehicle (potassium bicarbonate buffer used to dissolve zop), was administered for three weeks through once daily oral gavage. AR activity and sorbitol measurements were performed as published earlier.
Experimenters were blinded to the genotype, diet and specific treatments. At the conclusion of the studies, data from experiments were analyzed by experimenters who were unaware of the experimental groups or treatments. For human subjects, researchers obtained cDNA samples of subcutaneous fat from fasted lean and fasted subjects with obesity.
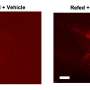
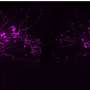
Results showed that after HFD feeding, a significant increase in AR and senescence marker Cdkn2a expression in all tissues compared to mice fed chow. Glycerol, non-esterified free fatty acid (NEFA) and triglycerides measured from plasma of these mice after a four-hour fast revealed significantly higher NEFA levels in HFD-fed instead chow-fed mice. Studies in cDNA samples from human subjects with obesity showed increased expression of AR and senescence marker.
Measurements of released NEFA indicated an approximately 50% reduction in β3-agonist CL 316,423 (CL)-stimulated lipolysis of the scAT fat pads retrieved from HFD-fed mice compared to standard chow-fed animals; no diet-dependent differences in basal lipolysis were observed. AR knockout mice and ARI treated mice on HFD showed reduced senescence and increased lipolysis in scat. Taken together, these results indicate evidence of increased scat senescence and defective CL stimulated lipolysis in mice with obesity is driven, in part, by AR.
"This is an important discovery, and the data is compelling. Today, we don't have good medicines that target dysfunctional adipose tissue. I'm very excited by this work; these findings should compel scientists to find drugs that impact this novel pathway and could be used to treat both obesity and diabetes," said AdventHealth Senior Vice President and Chief Scientific Officer Steven R. Smith, MD. An expert in this area, Smith was not associated with the research.
Other authors of the study include Devi Thiagarajan, Nosirudeen Quadri, Shabnam Jawahar, Hylde Zirpoli, Carmen Hurtado del Pozo, Raquel López-Díez, Syed Nurul Hasan, Gautham Yepuri, Paul F. Gugger, and Ann Marie Schmidt of the Diabetes Research Program, Department of Medicine, NYU Grossman School of Medicine, New York; Brian S. Finlin and Philip A. Kern of the Center for Clinical and Translational Sciences, University of Kentucky and Kenneth Gabbay, Baylor College of Medicine, Houston, Texas. Thiagarajan was also with the Saha Cardiovascular Research Center, Department of Physiology, University of Kentucky.
The study, titled "Aldose Reductase Promotes Diet-induced Obesity via Induction of Senescence in Subcutaneous Adipose Tissue," will be published in the August 2022 print issue.
More information: Aldose Reductase Promotes Diet-induced Obesity, Obesity, 2022. onlinelibrary.wiley.com/doi/10.1002/oby.23496