Aging, diet-induced obesity, and metabolic disease link explored in new research
Unraveling the links among obesity, aging, telomere lengths and metabolic diseases is the subject of the study published today in Nature Metabolism by a collaborative research team at The University of Texas Health Science Center at Houston (UTHealth).
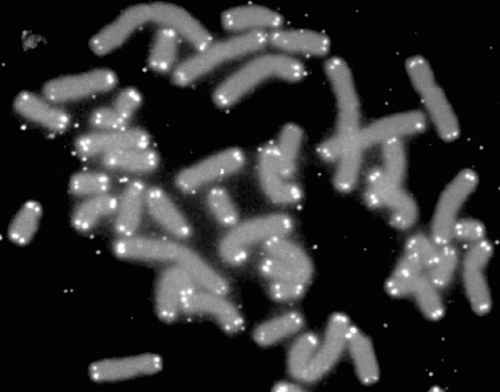
Telomeres act as protective caps at the end of chromosomes to prevent them from replication errors during cell divisions. Every time a chromosome replicates itself, telomeres shorten. When the telomeres become too short, the cell can no longer replicate its chromosomes safely and becomes arrested, or senescent. That shortening has been linked to the aging process and development of degenerative diseases.
"Recent studies have also shown the connection between obesity-induced metabolic diseases, such as Type 2 diabetes, and the accumulation of senescent cells, which entered the state of irreversible proliferation arrest," said lead author Mikhail Kolonin, Ph.D., professor and Harry E. Bovay, Jr. Distinguished University Chair in Metabolic Disease Research with McGovern Medical School at UTHealth. "Cell senescence can be caused by telomere shortening due to excessive stem cell division."
It has been unclear how excessive feeding and obesity are linked with cell senescence mechanistically. The authors hypothesized that overfeeding, causing excessive stem cell proliferation and telomere attrition, predisposes adipose (fat) cells to premature senescence and tissue dysfunction.
"Until now we have not been able to investigate the process in laboratory mice because they have abnormally long telomeres that do not shorten sufficiently to trigger replicative senescence within their lifespan," said Kolonin, who is the director of the Center for Metabolic and Degenerative Diseases at the Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases at McGovern Medical School.
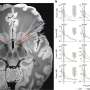
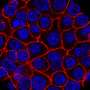
Telomere shortening is prevented by telomerase, an enzyme rebuilding the telomeres at each cell division. The group created mice with telomerase genetically inactivated in stem cells giving rise to adipocytes, the lipid-storing cells of fat tissue. Simulating clinical observations, these mice underwent replicative senescence in fat tissue, further advanced by high-calorie diet, and developed Type 2 diabetes.
To further assess translational relevance of these findings, the group analyzed biopsies from patients undergoing bariatric surgery. Shorter telomeres were observed in patients who were more resistant to the weight loss effects of the treatment and had a history of metabolic dysfunction. Combined, these results provide a new insight on the mechanistic links between aging, diet-induced obesity, and metabolic disease.
More information: Gao, Z., Daquinag, A.C., Fussell, C. et al. Age-associated telomere attrition in adipocyte progenitors predisposes to metabolic disease. Nat Metab 2, 1482–1497 (2020). doi.org/10.1038/s42255-020-00320-4