Tackling resistance to HIF2 drugs with an RNA-based therapy
Expected to be diagnosed in 2% of men and 1% of women in the U.S., kidney cancer has traditionally been one of the most challenging cancers to treat. Until 2005, only one drug had been approved by the Food and Drug Administration (FDA) to treat kidney cancer, which is resistant to both chemotherapy and conventional radiation. Since then, discoveries about its biology have led to a flurry of targeted and immune therapies. But despite these advances, still today, most patients succumb to the disease once the cancer has spread.
Discovered at UT Southwestern Medical Center (UTSW), the HIF2α protein is the most important driver of kidney cancer, which has traditionally been regarded as undruggable. UTSW scientists identified a vulnerability in the HIF2α protein structure and identified chemicals blocking HIF2α. Licensed to Peloton Therapeutics in the UTSW BioCenter, these chemicals set the foundation for the development of PT2977 (also called belzutifan), which received FDA approval for the treatment of hereditary kidney cancer in 2021, and is currently being evaluated against nonfamilial kidney cancer in multiple phase 3 clinical trials by Merck, which acquired Peloton.
However, studies in mice and subsequently in patients have shown that kidney tumors develop resistance. Resistance arises as a result of mutations, including mutations that block the attachment of the drug to HIF2α.
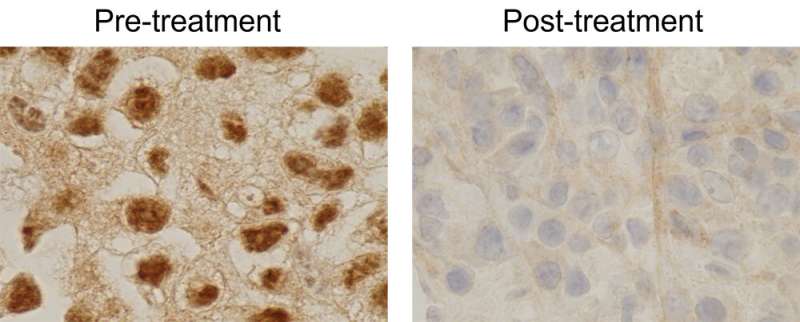
For the last seven years, scientists in the Brugarolas lab at UTSW have partnered with Arrowhead Pharmaceuticals to develop a second-generation inhibitor with activity not only against unmodified but also mutated HIF2α. Using the same platform of patient tumors transplanted in mice that provided the first evidence of anti-cancer activity by Peloton's HIF2α blocking drugs, the authors now show that this second-generation drug is also active against kidney cancer. Proof of principle of activity is also provided in humans from the phase 1 trial led by James Brugarolas, M.D., Ph.D., Professor of Internal Medicine and Director of the Kidney Cancer Program at the Harold C. Simmons Comprehensive Cancer Center of UTSW.
Like the FDA-approved Pfizer-BioNTech and Moderna COVID-19 vaccines, this second-generation HIF2α inhibitor (ARO-HIF2) is an RNA-based drug. ARO-HIF2 is a dsRNA drug that blocks the production of HIF2α in cancer cells. A particular advantage of ARO-HIF2 is the presence of a "homing device" that specifically targets it to cancer cells by interacting with a protein on their surface.
"RNA-based medicines hold promise to treat many different diseases, as evidenced by a recent string of new FDA approvals. However, the unsolved challenge in cancer is delivery. Developing delivery systems to target tumor cells, something that was accomplished with ARO-HIF2, is an area of expanding research," said Daniel Siegwart, Ph.D., associate professor of biochemistry at UTSW and a member of the Simmons Cancer Center.
The studies were published in Clinical Cancer Research and the Journal of Clinical Oncology.
More information: Yuanqing Ma et al, HIF2 inactivation and tumor suppression with a tumor-directed RNA-silencing drug in mice and humans, Clinical Cancer Research (2022). DOI: 10.1158/1078-0432.CCR-22-0963
James Brugarolas et al, Initial results from the phase 1 study of ARO-HIF2 to silence HIF2-alpha in patients with advanced ccRCC (AROHIF21001)., Journal of Clinical Oncology (2022). DOI: 10.1200/JCO.2022.40.6_suppl.339