Finding the principle regulating the secretion of neurotransmitters, an important clue to brain disease treatment
Professor Suh Byung-chang's research team of the Department of Brain Sciences, DGIST, announced on Wednesday, November 23 that they have identified the molecular mechanism for the activation of "voltage-dependent calcium channels," an important protein that regulates the secretion of neurotransmitters at nerve cell terminals.
Developing a system that regulates only the activity of specific voltage-dependent calcium channels is expected to provide clues to new research that can treat neurological diseases such as various mental illnesses and chronic pains.
Among the voltage-dependent calcium channels that regulate the inflow of calcium ions, the "CaV2.2" channel plays an essential role in signal transmission between nerve cells, expressed at the axon terminal of nerve cells, to regulate the secretion of neurotransmitters. It has been reported that mental illnesses such as bipolar disorder, schizophrenia, autism, epilepsy, and chronic pain occur when there is a problem in regulating the activity of CaV2.2.
The cell membrane is formed by substances called phospholipids. PIP2, which is a phospholipid, is known to be important for the activity of voltage-dependent calcium channels. However, the question on how PIP2 regulates calcium channel activity has not been clearly identified due to the complex structure in which several subunits are bound.
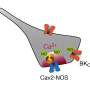
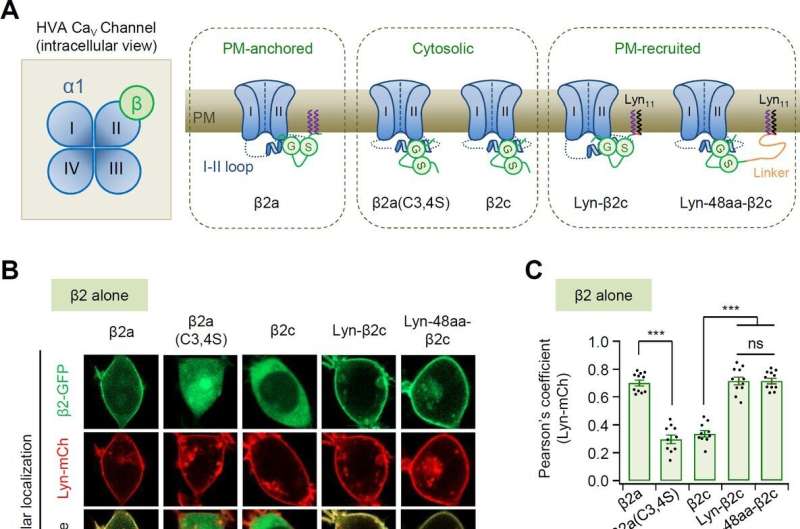
Professor Suh Byung-chang's research team studied the molecular mechanism of PIP2 concerning the activation of various receptors and ion channels. In particular, the research team clarified that the sensitivity of the calcium channel to PIP2 varies depending on whether the β2 unit, an auxiliary subunit of the voltage-dependent calcium channel, is bound to the cell membrane.
Based on these studies, this study attempted to identify the principle in how PIP2 regulates the activity of CaV2.2 differently depending on whether the β2 unit is bound to the cell membrane at the molecular level. The research team created various mutation models of CaV2.2 channels and β2 units through genetic recombination and confirmed them using electrophysiological techniques.
As a result, it was found that PIP2 binds to the "I-II loop," to which β2 units bind, in the CaV2.2 channel and to "S4II," one of the voltage sensing domains, respectively, to regulate the activity of the channel. In addition, it was found that the binding of PIP2 to the I-II loop is determined depending on whether the β2 unit binds to the cell membrane, thereby regulating the activity of CaV2.2.
Furthermore, it was confirmed that CaV2.2 activity can be regulated in real-time by developing a system that can artificially control the binding of PIP2 to the I-II loop of CaV2.2 by applying β2 unit.
The result identified a new action mechanism of the CaV2.2 channel activity, which plays a vital role in signal transmission between nerve cells. It is expected to provide an important clue to the treatment of mental disorders, such as autism, bipolar disorder, and schizophrenia and of fatal neurological diseases, such as epilepsy and chronic pain in the future.
In this study, Dr. Park Chun-Kyu of the Department of Brain Sciences, DGIST, participated as the first author, and Professor Suh Byung-chang participated as the corresponding author. The study results were published in eLife.
More information: Cheon-Gyu Park et al, Molecular basis of the PIP2-dependent regulation of CaV2.2 channel and its modulation by CaV β subunits, eLife (2022). DOI: 10.7554/eLife.69500