This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Toward a personalized approach to the study and treatment of bone cancers
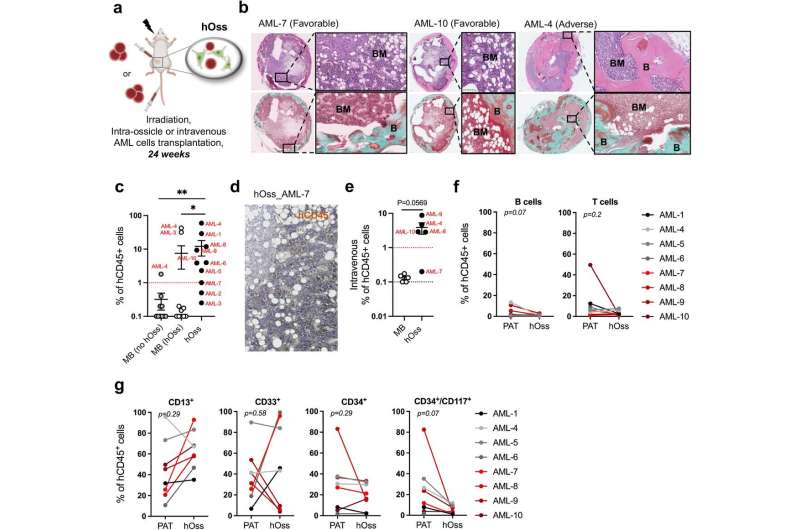
Researchers at Lund University have generated human mini bones in the lab which mirror the composition and function of human bone. The results published in Science Translational Medicine detail this step toward the future development of patient-tailored, personalized models of bone cancers and tumors.
On average, the adult body consists of 206 bones. Housed in the center of each, is bone marrow, the spongy substance which manufactures stem cells and other substances needed to produce a variety of blood cells. This microenvironment serves a vital purpose, supporting blood production throughout our lifetime. While a perfect place for blood cells to grow and mature, it can also become a hotspot for blood disorders like leukemia to form and other cancers, such as prostate or breast cancer, to metastasize or spread in their later stages.
Today, several treatment options are available to help patients to combat these conditions, though how a patient responds to treatment tends to vary greatly. Thus, new innovative therapies which are tailored to the individual and target the right treatments, to the right patients, at the right time are needed going forward. Key to bringing these treatment options into the 21st century are personalized models of cancer which reflect each patient's condition.
"Currently, studying the processes of bone and blood cell formation in the human body and various cancers developing in bone is a real challenge. Existing models are mainly in mice and do not fully reflect the human condition. This impairs what we can learn about these diseases and limits the development of efficient therapies," says Paul Bourgine, Assistant Professor and Wallenberg Center for Molecular Medicine Fellow.
For that reason, he and his research team within the Laboratory of Bone Organ Modeling and Regeneration have set to work to develop a preclinical tool to model bone-developing tumors using a patient's cells. Their latest study propels their research forward, detailing how they have generated human ossicles or mini bones in the lab, which mirror the composition and function of human bone.
"We provide a new, robust tool which can be used to study bone and bone marrow formation and also investigate the development of bone-developing cancers, such as leukemia or even solid tumors that ultimately metastasize to bones," says Paul Bourgine.
The team of scientists, including first-author of the study, Dr. Ani Grigoryan, genetically engineered mesenchymal stem cells—adult stem cells isolated from different sources, including bone marrow, that can become other types of cells—and guided them to follow a developmental program like the embryonic process through which our bones naturally form.
These cells were then implanted into mice models, which led to the formation of mature mini-bones and bone marrow tissues. Later, these same stem cells were found in the generated mini-bones where they had differentiated into several types of cells critical to support the growth and development of both healthy human cells and cancer cells.
"In the short term, this will be extremely valuable to understand the interactions between human cancer cells and their microenvironment and how cancer cells modify this environment to favor their survival and progression. Answering these questions will be key to developing and testing new therapeutics. Ultimately, we trust that the human mini-bone technology can be used in the future as an advanced drug-screening platform," says Paul Bourgine.
New research is already underway where Paul Bourgine and his team are using this technology to develop a personalized approach to the study and treatment of bone-developing tumors by generating these mini bones from a patient's own cells.
More information: Ani Grigoryan et al, Engineering human mini-bones for the standardized modeling of healthy hematopoiesis, leukemia, and solid tumor metastasis, Science Translational Medicine (2022). DOI: 10.1126/scitranslmed.abm6391. pubmed.ncbi.nlm.nih.gov/36223446/