This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Novel therapeutic targets discovered for triple-negative breast cancer
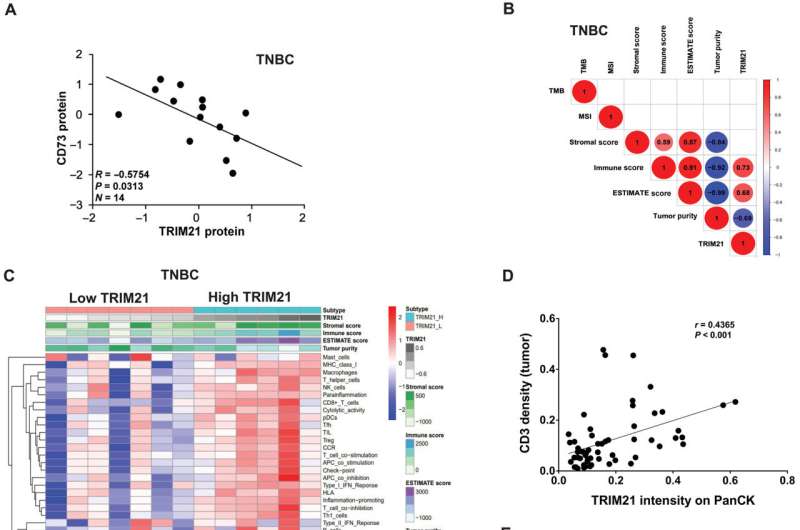
Targeting cellular post-transcription mechanisms in the CD73 ectoenzyme may promote anti-tumor immunity and slow cancer progression in triple-negative breast cancer, according to a Northwestern Medicine study published in Science Advances.
The study, co-led by Bin Zhang, MD, Ph.D., professor of Medicine in the Division of Hematology and Oncology and of Microbiology-Immunology, suggests a new immunotherapy strategy for patients who currently lack effective treatment options.
"For triple-negative breast cancer, you want to consider targeting a major immunosuppressive mechanism, and targeting CD73 has now become an emerging option in addition to other conventional checkpoint blockades," said Zhang, who is also co-leader of the Tumor, Environment & Metastasis (TEAM) Program at the Robert H. Lurie Comprehensive Cancer Center of Northwestern University.
Triple-negative breast cancer (TNBC) cells do not contain the typical hormone and protein receptors commonly found in breast cancer cells, which leaves a limited number of viable therapeutic targets.
In addition to surgery, radiation and chemotherapy, immunotherapies such as immune checkpoint inhibitors—drugs that identify and block specific proteins or "checkpoints" produced by immune cells and cancer cells—have been widely used to treat solid tumors, including TNBC. However, previous clinical trials have shown that most patients with TNBC have little to no response to that kind of therapy.
In the current study, the investigators aimed to identify new therapeutic targets that can mobilize the body's immune system to overcome tumor-induced immunosuppression from TNBC cells.
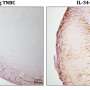
By analyzing TNBC cell lines, the team discovered that elevated levels of the active ectoenzyme CD73 were expressed on the surface of cancer cells. This increased expression of CD73 is abnormal, according to Zhang, suggesting that elevated levels of the enzyme increase immunosuppressive activity within the tumor microenvironment.
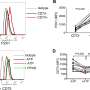
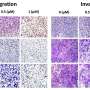
Using advanced microscopy techniques to investigate the cancer cells further, the investigators found that the ubiquitinase protein TRIM21 mediates the degradation of CD73, and disrupting TRIM21 stabilized CD73 and, in turn, suppressed CD8-positive T-cells that would have otherwise promoted an adaptive immune response against the cancer.
"Therefore, you can actually provide additional options to generate reagents to block the structural interaction between these two molecules," Zhang said.
The investigators also extracted specific amino acids from CD73, which degraded essential intracellular functions of CD73, specifically ubiquitylation, and enhanced tumor growth by preventing antitumor immunity.
Overall, the findings reveal a new potential therapeutic strategy in which mitigating CD73 protein levels could prevent TNBC tumor progression.
Decreased levels of CD73 and increased levels of TRIM21 in cancer cells could also serve as biomarkers for identifying patients who may have a more favorable response to immunotherapy, according to Zhang.
"We think if you modulate CD73 protein levels directly, not only can you diminish the enzyme activity but also can target CD73 independent of enzyme activity function," Zhang said.
More information: Ziyi Fu et al, Proteolytic regulation of CD73 by TRIM21 orchestrates tumor immunogenicity, Science Advances (2023). DOI: 10.1126/sciadv.add6626. www.science.org/doi/10.1126/sciadv.add6626