This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study offers a potential strategy to improve T cell therapy in solid tumors
A new approach that delivers a "one-two punch" to help T cells attack solid tumors is the focus of a preclinical study by researchers from the Perelman School of Medicine at the University of Pennsylvania. The findings, published in the Proceedings of the National Academy of Sciences (PNAS), showed that targeting two regulators that control gene functions related to inflammation led to at least 10 times greater T cell expansion in models, resulting in increased antitumor immune activity and durability.
CAR T cell therapy was pioneered at Penn Medicine by Carl H. June, MD, the Richard W. Vague Professor in Immunotherapy at Penn and director of the Center for Cellular Immunotherapies (CCI) at Abramson Cancer Center, whose work led to the first approved CAR T cell therapy for B-cell acute lymphoblastic leukemia in 2017. Since then, personalized cellular therapies have revolutionized blood cancer treatment, but remained stubbornly ineffective against solid tumors, such as lung cancer and breast cancer.
"We want to unlock CAR T cell therapy for patients with solid tumors, which include the most commonly diagnosed cancer types," said June, the new study's senior author. "Our study shows that immune inflammatory regulator targeting is worth additional investigation to enhance T cell potency."
One of the challenges for CAR T cell therapy in solid tumors is a phenomenon known as T cell exhaustion, where the persistent antigen exposure from the solid mass of tumor cells wears out the T cells to the point that they aren't able to mount an antitumor response. Engineering already exhausted T cells from patients for CAR T cell therapy results in a less effective product because the T cells don't multiply enough or remember their task as well.
Previous observational studies hinted at the inflammatory regulator Regnase-1 as a potential target to indirectly overcome the effects of T cell exhaustion because it can cause hyperinflammation when disrupted in T cells—reviving them to produce an antitumor response. The research team, including lead author David Mai, a Bioengineering graduate student in the School of Engineering and Applied Science, and co-corresponding author Neil Sheppard, DPhil, head of the CCI T Cell Engineering Lab, hypothesized that targeting the related but independent Roquin-1 regulator at the same time could boost responses further.
"Each of these two regulatory genes has been implicated in restricting T cell inflammatory responses, but we found that disrupting them together produced much greater anticancer effects than disrupting them individually," Mai said. "By building on previous research, we are starting to get closer to strategies that seem to be promising in the solid tumor context."
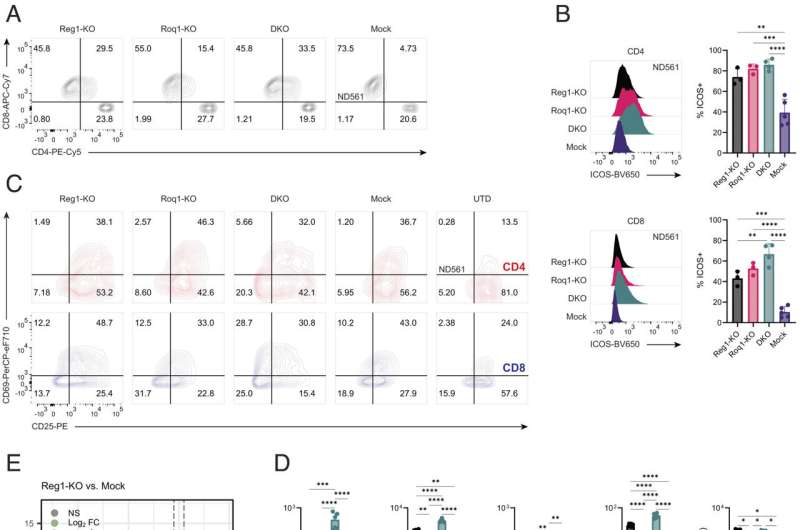
The team used CRISPR-Cas9 gene editing to knock out Regnase-1 and Roquin-1 individually and together in healthy donor T cells with two different immune receptors that are currently being investigated in Phase I clinical trials: the mesothelin-targeting M5 CAR (mesoCAR) and the NY-ESO-1-targeting 8F TCR (NYESO TCR). Neither engineered T cell product targets CD19, the antigen targeted by most approved CAR T cell therapies, as this antigen is not present in solid tumors.
After CRISPR editing, the T cells were expanded and infused in solid tumor mice models, where researchers observed the double knockout led to at least 10 times as many engineered T cells compared to disabling Regnase-1 alone, as well as increased antitumor immune activity and longevity of the engineered T cells. In some mice, it also led to overproduction of lymphocytes, causing toxicity.
"CRISPR is a useful tool for completely ablating the expression of target genes like Regnase and Roquin, resulting in a clear phenotype, however there are other strategies to consider for translating this work to the clinical setting, such as forms of conditional gene regulation," Sheppard said.
"We're certainly impressed by the antitumor potency that was unleased by knocking out these two non-redundant proteins in combination. In solid tumor studies, we often see limited expansion of CAR T cells, but if we're able to make each T cell more potent, and replicate them to greater quantities, we expect T cell therapies to have a better shot at attacking solid tumors."
Additional authors include Omar Johnson, Jordan Reff, Ting-Jia Fan, and John Scholler.
More information: David Mai et al, Combined disruption of T cell inflammatory regulators Regnase-1 and Roquin-1 enhances antitumor activity of engineered human T cells, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2218632120