This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
YAP/TEAD inhibitor VT3989 is well tolerated, shows antitumor activity in advanced mesothelioma and NF2-mutant cancers
The first-in-class YAP/TEAD inhibitor VT3989 was well tolerated with durable antitumor responses in patients with advanced malignant mesothelioma and other tumors with NF2 mutations, according to results of a Phase I trial led by researchers at The University of Texas MD Anderson Cancer Center. The first-in-human study was presented today at the American Association for Cancer Research (AACR) Annual Meeting 2023.
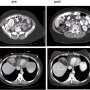
Seven of 69 patients had radiological partial responses that persisted up to at least 21 months, indicating tumor shrinkage, while 34 had stable disease. Patient benefit was observed in patients with both mesothelioma and other solid tumors, including those with and without NF2 mutations.
"This is the first clinical proof-of-concept for drugging the Hippo-YAP-TEAD pathway," said presenter and lead author Timothy A. Yap, M.D., Ph.D., associate professor of Investigational Cancer Therapeutics. "We've long known that YAP is a key oncogenic driver of cancer, and preclinical research suggested blocking YAP could shrink tumors. However, this is the first data demonstrating that targeting YAP can work in a clinical setting to actually benefit patients."
YAP (yes-associated protein) is a transcriptional co-activator that works with TEAD (transcriptional enhancer activator domain) as a part of the Hippo signaling pathway, which is important for normal cell growth and regulating the immune response. However, in several cancer types, YAP is either overexpressed or over-activated due to dysfunction in the pathway, fueling cancer cell growth. Therefore, YAP represents an attractive therapeutic target.
VT3989 works by inhibiting TEAD palmitoylation, which in turn blocks YAP function. NF2-mutant cancers were chosen for this trial due to their dependence on YAP activity for growth.
The Phase I dose escalation trial was designed to evaluate the safety, tolerability and recommended Phase II dose for VT3989. Today's presentation includes data on 69 enrolled patients, including 43 with advanced malignant mesothelioma and 26 with other solid tumors. Patients were heavily pre-treated and received a median of three prior lines of therapy. A total of 37 patients had NF2 mutations. Patients in the trial were 51% male and 49% female, 87% white, 10% Hispanic and 3% Black, with a median age of 63.5.
The drug was well tolerated, with no dose-limiting toxicities observed. The most common adverse events were albuminuria (a reversible increase in the protein albumin in the urine), swelling in the extremities, fatigue and nausea. Only seven grade 3 adverse events were possibly treatment-related, including albuminuria, swelling, fatigue, and increased liver enzymes alanine transaminase and aspartate aminotransferase, along with one possibly related grade 4 cardiomyopathy.
"These are early but very promising results showing that this previously 'undruggable' target and anti-cancer strategy can work in a clinical setting," Yap said. "We look forward to future results from this study in order to evaluate whether this approach may bring an urgently needed treatment option to these patients."
Ongoing dose optimization cohorts of the study are investigating different dosing and scheduling in two-stage designs.
More information: Abstract: www.abstractsonline.com/pp8/#! … 8/presentation/10247