This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers synthesize chimeric peptide that elicits antitumor activity for cancer immunotherapy
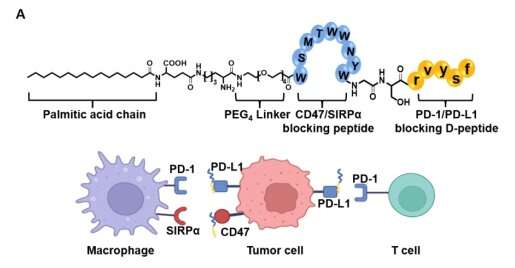
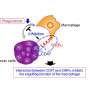
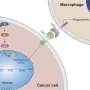
Professor Yanfeng Gao's team from the School of Pharmaceutical Sciences (Shenzhen), Sun Yat-sen University have designed and synthesized Pal-DMPOP, a chimeric peptide that can simultaneously block CD47/SIRPα and PD-1/PD-L1. This bispecific peptide elicits synergistic antitumor activity by enhancing macrophages phagocytosis and activating CD8+ T cells. The findings are published in the journal Science China Life Sciences.
Although immune checkpoint inhibition has been shown to effectively activate antitumor immunity in various tumor types, only a small subset of patients can benefit from PD-1/PD-L1 blockade. CD47 expressed on tumor cells protects them from phagocytosis through interaction with SIRPα on macrophages, while PD-L1 dampens T cell-mediated tumor killing.
Also, it was reported that macrophages can also express PD-1 to mediate a "don't eat me" signal through interaction with PD-L1 on tumor cells. Compared with antibodies, peptides have better tumor penetration ability and easy to be synthesized. Therefore, design of peptides dual-targeting blockade of both PD-1/PD-L1 and CD47/SIRPα may improve the efficacy of cancer immunotherapy.
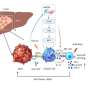
In this article, the authors designed a chimeric peptide Pal-DMPOP. It consists of the smallest fragment of the D-peptide inhibitor of PD-1/PD-L1 (OPBP-1) and the optimized peptide inhibitor of CD47/SIRPα (Pep-20). Also, the palmitic acid tail was modified at its N-terminal to improve its anti-enzymatic ability and in vivo half-life.
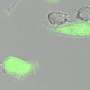
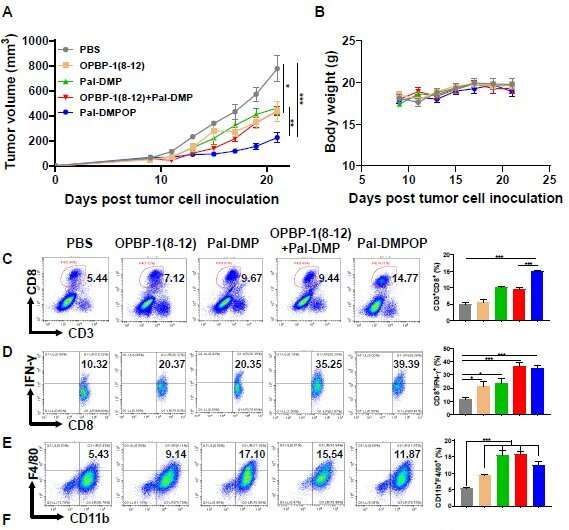
The research team verified that Pal-DMPOP can improve the phagocytosis of macrophages on tumor cells, and can also restore the killing effect of CD8+T cells on tumor cells in vitro. The effect of tumor immunotherapy in vivo has been determined in MC38 and CT26 mouse models. The tumor volume in Pal-DMPOP administration group is significantly reduced compared with the control group, and Pal-DMPOP has no obvious toxic effect in the tumor-bearing mice.
More information: Zheng Hu et al, Design of a novel chimeric peptide via dual blockade of CD47/SIRPα and PD-1/PD-L1 for cancer immunotherapy, Science China Life Sciences (2023). DOI: 10.1007/s11427-022-2285-6