This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
KRAS mutation shown to promote tumor evasion of innate immune surveillance in lung cancer
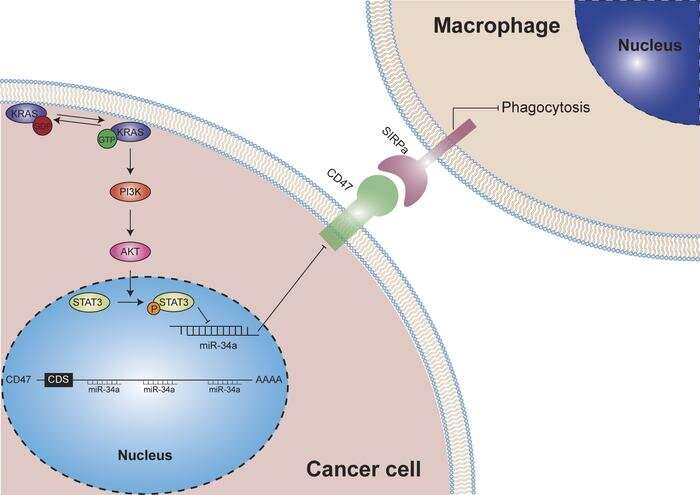
KRAS is one of the most frequently activated oncogenes in human cancers. While the role of KRAS mutation in tumorigenesis and tumor maintenance has been extensively studied, the relationship between KRAS and the tumor immune microenvironment is not fully understood. In this study, the researchers identified a novel role of KRAS in driving tumor evasion from innate immune surveillance.
In lung adenocarcinoma patient samples and Kras-driven genetic mouse models of lung cancer, mutant KRAS activates the expression of CD47, an antiphagocytic signal in cancer cells, leading to decreased phagocytosis of cancer cells by macrophages. Mechanistically, mutant KRAS activates PI3K-STAT3 signaling, which restrains miR-34a expression and relieves the post-transcriptional repression of miR-34a on CD47.
In three independent lung cancer patient cohorts, the researchers showed that KRAS mutation status positively correlates with CD47 expression.
Therapeutically, disruption of the KRAS-CD47 signaling axis with KRAS siRNA, the KRAS(G12C) inhibitor AMG 510 or miR-34a mimic suppresses CD47 expression, enhances the phagocytic capacity of macrophages and restores innate immune surveillance. Overall, this study reveals a direct mechanistic link between active KRAS and innate immune evasion and identifies CD47 as a major effector underlying KRAS-mediated immunosuppressive tumor microenvironment.
The scientific merit of this study lies in the following aspects:
- It identifies a previously unsuspected role of KRAS in evading innate immune surveillance. The findings of this study, complement with previous findings of the role of KRAS in adaptive immune response, indicate that mutant KRAS lies at the heart of tumor immune evasion. Inhibition of KRAS signaling can therefore render cancer cells more susceptible to immune attack by both T cells (adaptive immunity) and macrophages (innate immunity), which might contribute to the overall anti-tumor effect of KRAS inhibitors in vivo.
- It establishes a positive correlation between KRAS mutation status and CD47 expression in lung adenocarcinoma patients. Anti-CD47 antibodies have recently entered phase III clinical trials yet predictive biomarkers of therapeutic response are still lacking. KRAS mutation status may serve as a biomarker for patient selection to enhance the effectiveness of anti-CD47 immunotherapy.
- It provides initial preclinical evidence supporting the combination therapy using KRAS inhibitors and anti-CD47 antibodies. Clinical trials combining the KRAS(G12C) inhibitors AMG510/MRTX849 and PD1/PD-L1 inhibitors are already underway. It's very likely that the combination of KRAS inhibitors and anti-CD47 antibodies may provide synergistic benefits to patients with KRAS-driven cancers.
The paper is published in the Journal of Clinical Investigation.
More information: Huanhuan Hu et al, Oncogenic KRAS signaling drives evasion of innate immune surveillance in lung adenocarcinoma by activating CD47, Journal of Clinical Investigation (2022). DOI: 10.1172/JCI153470