This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Mutant KRAS regulates Y chromosome gene in colorectal cancer, driving metastasis and inhibiting immunity

Researchers at The University of Texas MD Anderson Cancer Center have uncovered a gene on the Y chromosome that is upregulated in KRAS-mutated colorectal cancer (CRC), increasing tumor cell invasiveness and reducing anti-tumor immunity in male patients.
The preclinical study, published today, June 21, in Nature, provides novel insights into the longstanding mystery of molecular and cellular mechanisms that drive increased metastasis and poor prognosis in men with CRC. The results highlight the Y chromosome gene KDM5D, which codes for an epigenetic enzyme, as a potential therapeutic target and uncover its non-canonical function in regulating anti-tumor immune responses.
"Harnessing the experimental merits of novel colorectal cancer models, our integrated computational and functional analyses revealed that oncogenic KRAS regulates a Y chromosome gene that promotes metastasis and tumor immune evasion in males," said corresponding author Ronald DePinho, M.D., professor of Cancer Biology. "We now have an actionable target meriting further investigation, providing a path to intercept that will change the natural history of the disease in men with KRAS-mutant colorectal cancer."
There have long been prominent sex differences in the outcomes of patients with metastatic CRC—the second most common cause of cancer death—with males typically having higher incidences and worse prognoses than females. However, the underlying mechanisms for these differences have been mainly attributed to lifestyle differences and possibly sex hormones.
While lifestyle changes can benefit patients, many clinical studies evaluating hormonal interventions to improve male CRC outcomes have yielded negative or inconclusive results, highlighting a need for a deeper mechanistic understanding of the molecular nuances that drive more aggressive disease in men.
Laboratory models reveal sex-based differences in tumor biology
First author Jiexi Li, Ph.D., of the DePinho laboratory, led the research effort and generated genetically engineered mouse models that closely mirror the evolution of human CRC metastatic progression, including models with oncogenic KRAS as well as inactivated APC and p53 tumor suppressors, the most commonly mutated proteins found in patients with CRC.
Li, a recent graduate of the MD Anderson UTHealth Houston Graduate School of Biomedical Sciences, observed that only models with the KRAS mutation showed sex-specific differences, with males exhibiting a higher frequency of metastasis and shorter overall survival compared to females. This led Li to hypothesize that KRAS was the likely driver of these sex-based disparities.
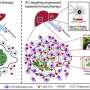
Cross-species and transcriptomic analyses revealed KDM5D was highly upregulated in these models, and further exploration uncovered its significant involvement in repressing genes governing cell adhesion and anti-tumor immunity, promoting metastatic behavior.
KDM5D epigenetically disrupts cell junctions, allowing for transition to metastasis
One of the earliest steps in metastasis is the loss of cell-cell junctions, which allows cancer cells to transition into a migratory state. The researchers discovered the cell junction gene AMOT was downregulated in the KRAS-mutated metastatic cancer models.
Additionally, in patient samples, there was a negative correlation between AMOT and KDM5D expression in tumors from males; AMOT expression was lower in KRAS-mutant CRC cell lines from males than from females. Further analysis showed that KDM5D epigenetically represses AMOT, impairing these cell junctions and enabling cancer cells to shift into a metastatic state.
Deleting KDM5D in the models restored AMOT levels in cancer cells from laboratory models and from CRC patients, repairing cell junctions and decreasing cancer cell invasiveness. Conversely, using a transgene to enforce KDM5D expression promoted tumor invasion in the absence of oncogenic KRAS.
KDM5D's novel function hinders recognition of cancer cells by the immune system
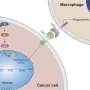
The researchers noted that metastatic cancer cells showed decreased activity of specific genomic elements called super-enhancers, which are involved in activating transcription of certain gene clusters. Specifically, there was lower expression of genes involved in antigen presentation through major histocompatibility complex I (MHC-1), the key mechanism of tumor recognition by the immune system. Previous studies have shown that MHC deficiency can facilitate tumor immune escape.
The study revealed that KDM5D represses the TAP1 and TAP2 genes, which normally assist in processing and presenting antigens that signal for T cells to eliminate abnormal cells. Therefore, KDM5D works in this setting to enable cancer cells to avoid detection and destruction from immune cells, promoting their ability to progress and metastasize.
"Not only does this explain sex-specific differences, but our work uncovered an entirely new biochemical function for histone demethylases in regulating histone acetylation at super-enhancers controlling immune system genes," DePinho said.
More information: Ronald DePinho, Histone demethylase KDM5D upregulation drives sex differences in colon cancer, Nature (2023). DOI: 10.1038/s41586-023-06254-7. www.nature.com/articles/s41586-023-06254-7