This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New genetic clues uncovered in largest study of families with multiple children with autism
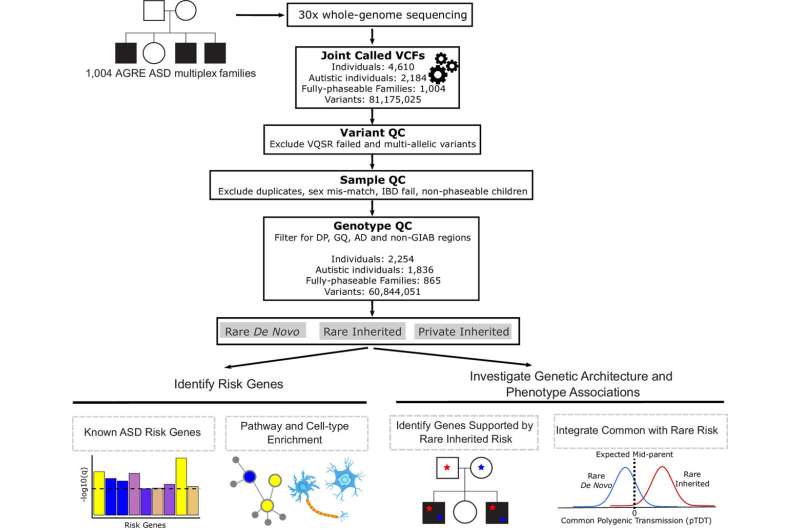
UCLA Health researchers have published the largest-ever study of families with at least two children with autism, uncovering new risk genes and providing new insights into how genetics influence whether someone develops autism spectrum disorder.
The new study, published in the Proceedings of the National Academy of Sciences, also provides genetic evidence that language delay and dysfunction should be reconsidered as a core component of autism.
Most genetic studies of autism have focused on families with one child affected by the neurodevelopmental disorder, sometimes excluding families with multiple affected children. As a result, few studies have examined the role of rare inherited variation or its interaction with the combined effect of multiple common genetic variations that contribute to the risk of developing autism.
"Study design is critical and not enough attention has been paid to studying families with more than one affected child," said lead study author Dr. Daniel Geschwind, the Gordon and Virginia MacDonald Distinguished Professor of Human Genetics, Neurology and Psychiatry at UCLA.
Autism is highly heritable: It is estimated at least 50% of genetic risk is predicted by common genetic variation and another 15%–20% is due to spontaneous mutations or predictable inheritance patterns. The remaining genetic risk is yet to be determined.
For this study, researchers performed whole genome sequencing in 4,551 individuals from 1,004 families with at least two children diagnosed with autism. This group included 1,836 children with autism and 418 children without an autism diagnosis.
The researchers found seven potential genes that are predicted to increase the risk of autism: PLEKHA8, PRR25, FBXL13, VPS54, SLFN5, SNCAIP, and TGM1. This is remarkable because other studies have had to analyze much larger cohorts to identify a similar number of novel risk genes. This is because in this case, most of the new genes were supported by rare inherited DNA variations that were transmitted from parents to children with autism.
The researchers also examined polygenic risk, in which a combination of commonly found genetic variations can raise the likelihood of developing autism. They found children who inherit rare mutations from unaffected parents in combination with polygenic risk are more likely to have autism. This helps explains why parents who carry a single rare mutation may not show signs of autism even if their children do. It also lends support to the liability threshold model, a concept in behavioral genetics that holds there is an additive effect of genes that influences the probability that someone develops a certain trait.
In another important finding, children who had language delay had a higher likelihood of inheriting a polygenic score associated with autism, while there was not a similar relationship for children without language delays. This pattern was specific to autism and was not seen in other traits like educational attainment, schizophrenia or bipolar disorder, suggesting there's a link between the genetic risk for autism and language delay.
However, the most recent edition of the professional guidebook used by mental health providers to diagnose disorders—the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5)—does not consider language delay a core autism symptom, citing the variability in language ability among people with autism.
"This association of general risk for ASD that was strongest in those with language delay suggests that language is actually a core component of ASD. This finding needs to be replicated in larger cohorts, especially those recruited more recently under DSM-5," Geschwind said.
More information: Matilde Cirnigliaro et al, The contributions of rare inherited and polygenic risk to ASD in multiplex families, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2215632120