This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Understanding the metabolites underlying eye development
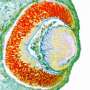
Aerobic glycolysis, the process by which cells transform glucose into lactate, is key for eye development in mammals, according to a new Northwestern Medicine study published in Nature Communications.
While it has been well known that retinal cells use lactate during cell differentiation, the exact role that this process plays in early eye development was not previously understood.
The findings further the field's understanding of the metabolic pathways underlying organ development, according to Guillermo Oliver, Ph.D., the Thomas D. Spies Professor of Lymphatic Metabolism, Director of the Feinberg Cardiovascular and Renal Research Institute Center for Vascular and Developmental Biology, and senior author of the study.
"For a long time, my lab has been interested in developmental biology. In particular, to characterize the molecular and cellular steps regulating early eye morphogenesis," Oliver said. "For us, the question was: 'How do these remarkable and critical sensory organs we have in our face start to form?'"
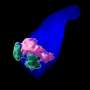
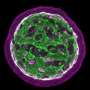
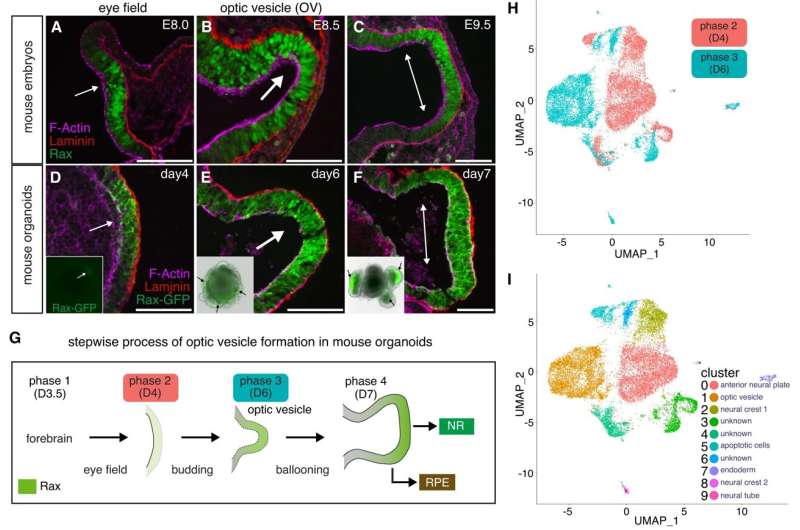
Nozomu Takata, Ph.D., a postdoctoral fellow in the Oliver lab and first author of the paper, initially approached this question by developing embryonic stem cell-derived eye organoids, which are organ-like tissues engineered in a petri dish. Intriguingly, he observed that early mouse eye progenitors display elevated glycolytic activity and production of lactate.
After introducing a glycolysis inhibitor to the cultured organoids, normal optic vesicle development halted, according to the study, but adding back lactate allowed the organoids to resume normal eye morphogenesis, or development.
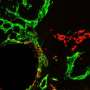
Takata and his collaborators then compared those organoids to controls using genome-wide transcriptome and epigenetic analysis using RNA and ChIP sequencing. They found that inhibiting glycolysis and adding lactate to the organoids regulated the expression of certain critical and evolutionary conserved genes required for early eye development.
To validate these findings, Takata deleted Glut1 and Ldha, genes known for regulating glucose transport and lactate production from developing retinas in mouse embryos. The deletion of these genes arrested normal glucose transport specifically in the eye-forming region, according to the study.
"What we found was an ATP-independent role of the glycolytic pathway," Takata said. "Lactate, which is a metabolite known as a waste product before, is really doing something cool in eye morphogenesis. That really tells us that this metabolite is a key player in organ morphogenesis and in particular, eye morphogenesis. I see this discovery as having broader implications, as likely also being required in other organs and maybe in regeneration and disease as well."
Following this discovery, Takata said he plans to continue to take advantage of traditional and emerging developmental biology's tools such as mouse genetics and stem cells-derived organoids to study the role of the glycolytic pathway and metabolism in the development of other organs.
The findings could also be useful in better understanding the direct effect that metabolites could have in regulating gene expression during organ regeneration and tumor development, Oliver said.
"Both regeneration and tumorigenesis involve developmental pathways that go awry in some occasions, or you need to reactivate," Oliver said. "For many developmental processes, you need very strict transcriptional regulation. A gene is on or off at certain times, and when that goes wrong, that could lead to developmental defects or promote tumorigenesis. Now that we know that there are specific metabolites responsible for normal or abnormal gene regulation, this can broaden our thinking on approaches to therapeutic treatments."
More information: Nozomu Takata et al, Lactate-dependent transcriptional regulation controls mammalian eye morphogenesis, Nature Communications (2023). DOI: 10.1038/s41467-023-39672-2