This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Combining immunotherapy with KRAS inhibitor eliminates advanced KRAS-mutant pancreatic cancer in preclinical models
Researchers at The University of Texas MD Anderson Cancer Center have uncovered a functional role for KRAS mutations in pancreatic cancer and rapidly translated these findings into a novel therapeutic approach combining a KRAS G12D inhibitor with immune checkpoint inhibitors for early- and late-stage KRAS G12D-mutant pancreatic cancer. The combination therapy led to durable tumor elimination and significantly improved survival outcomes in preclinical models, leading to the launch of a Phase I clinical trial.
Two studies, published in Developmental Cell and Cancer Cell, describe why KRAS-targeted monotherapy likely is not enough to completely eliminate tumors, suggesting that engaging the immune system is needed to prevent relapse. The comprehensive models generated for this investigation more accurately reflect the tumor microenvironment found in patients with metastatic disease, allowing for rigorous testing that provided unique insights into how oncogenic KRAS allows tumors to escape cancer cell death.
The studies were the result of collaborative work led by Krishnan Mahadevan, Ph.D., postdoctoral fellow in Cancer Biology, Kathleen McAndrews, Ph.D., instructor of Cancer Biology, and co-corresponding authors Raghu Kalluri, M.D., Ph.D., chair of Cancer Biology, Anirban Maitra, M.B.B.S., professor of Pathology and Translational Molecular Pathology, and Timothy Heffernan, Ph.D., vice president of Oncology Research for MD Anderson's Translational Research to AdvanCe Therapeutics and Innovation in ONcology (TRACTION) platform.
"By extensively testing the functional role of KRAS, we gained important insights into how better to prime the tumor microenvironment in advanced pancreatic cancer to improve treatment responses," Kalluri said. "These results are a testament to the value of team science and to the incredible research environment at MD Anderson, which enables the accelerated and seamless translation from genetic models to clinical application. We are encouraged that these results could lead to meaningful benefits for patients."
Pancreatic cancer is the third leading cause of cancer death in the United States and often is diagnosed at a late stage, when treatment options are limited and the prognosis is poor. KRAS G12D mutations occur in over 40% of pancreatic cancer cases, but KRAS inhibitors alone have not yielded durable responses for patients. Immunotherapy treatments also have not benefitted patients, partly due to an immunosuppressive tumor microenvironment in pancreatic tumors.
New models more accurately reflect tumor microenvironment
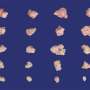
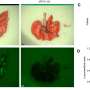
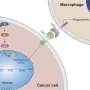
In the Developmental Cell study, researchers examined the functional role of KRAS by generating mouse models with various genetic alterations known to accompany KRAS mutations. Genetic suppression of KRAS in these models activated the Fas pathway, which is required for cancer cell death, and resulted in a greater number of T cells and fewer myeloid cells in the tumors.
This demonstrated that oncogenic KRAS epigenetically blocks expression of the Fas protein, silencing the pathway and allowing cancer cells to avoid an anti-tumor immune response. Suppressing KRAS led to complete tumor regression and significantly improved survival in these models, suggesting the potential for KRAS inhibition to improve immunotherapy responses and prevent relapse.
According to the authors, previous models did not adequately simulate the dynamic tumor microenvironment present in advanced pancreatic cancer. The current models helped to identify immune activation as a critical component for sustained anti-tumor effects, stressing the importance of having accurate preclinical models for producing fundamental basic science that can successfully translate into clinical applications.
KRAS G12D inhibition depends on immune cell activation
In the Cancer Cell study, the researchers built upon the first study by investigating the effects of a KRAS G12D inhibitor, MRTX1133, in 16 different lab models to determine its efficacy and safety in both early- and late-stage tumors.
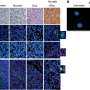
The study showed that KRAS G12D inhibition induced expression of the Fas pathway, reversed early tumor growth, increased CD8+ T cell infiltration, decreased myeloid infiltration and reprogrammed the tumor microenvironment in both early- and late-stage tumors. However, while initially successful, the tumors eventually grew back.
Further analysis showed that the elimination of established tumors was dependent upon the activation of CD8+ T cells. If CD8+ T cells were suppressed, the tumors progressed despite treatment with MRTX1133. Following this, the researchers found that combining various immune checkpoint inhibitors with MRTX1133 led to sustained tumor regression, enhanced cancer cell clearance and improved survival outcomes.
"These studies show that KRAS inhibition works, but monotherapy delivers only a transient response, especially when dealing with late-stage tumors," Maitra said. "Leveraging the immune system by combining KRAS inhibitors with immunotherapy was able to bring about the full effects of these drugs and provide the best possible survival benefits in our models."
The authors point out that these studies already have helped inform potential clinical applications for MRTX1133, and a Phase I clinical trial is ongoing at MD Anderson with contribution from the TRACTION research team.
The TRACTION platform, which was designed to overcome traditional hurdles in oncology drug development, incorporates a variety of cutting-edge technologies, disease modeling approaches and data analytics tools to quickly and safely advance the right treatments for the right patients. TRACTION is part of MD Anderson's Therapeutics Discovery division, an integrated team of clinicians, researchers and drug development experts working to advance impactful therapies for cancer.
More information: Raghu Kalluri, Elimination of oncogenic Kras in genetic mouse models eradicates pancreatic cancer by inducing Fas-dependent apoptosis by CD8+ T cells, Developmental Cell (2023). DOI: 10.1016/j.devcel.2023.07.025. www.cell.com/developmental-cel … 1534-5807(23)00394-5
Krishnan K. Mahadevan et al, KRASG12D inhibition reprograms the microenvironment of early and advanced pancreatic cancer to promote FAS-mediated killing by CD8+ T cells, Cancer Cell (2023). DOI: 10.1016/j.ccell.2023.07.002 cell.com/cancer-cell/fulltext/ … 1535-6108(23)00242-8