This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists develop new therapeutic model for potentially treating incurable eye diseases
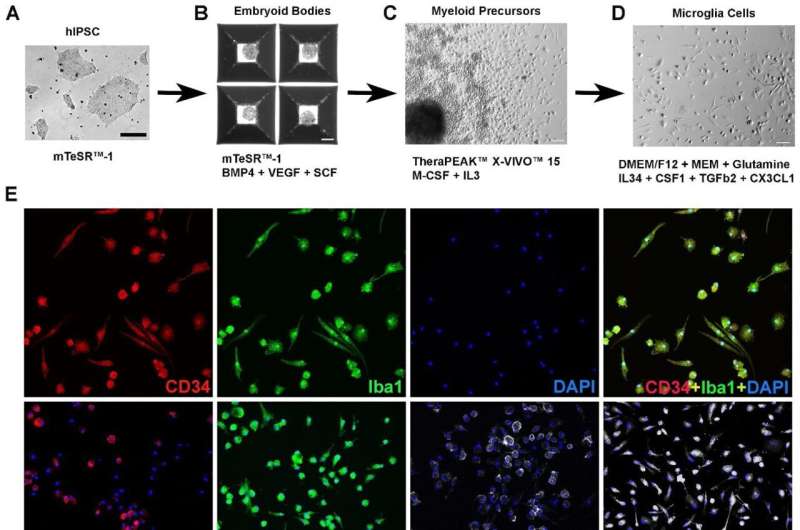
Researchers have successfully transplanted human microglia cells into mouse retina to create a model that could be used to test new treatments for incurable eye diseases.
The research, published today as a Reviewed Preprint in eLife, is described by the editors as an important study with solid data demonstrating the potential for microglial replacement therapy to treat retinal and central nervous system diseases.
Microglia are the innate immune cells of the central nervous system, which includes the retina, and play essential roles in the normal development of nerves and nerve connections (synapses). But they can also switch to a more sinister role—driving the development of brain and eye diseases, including age-related macular degeneration (AMD), glaucoma, diabetic retinopathy and uveitis (inflammation of the eye).
Under normal conditions, microglia vigilantly survey environmental changes to maintain normal retinal function. Under pathological conditions, they will respond quickly to injuries, but could also become inappropriately activated, grow in number, and migrate into surrounding tissues.
"Our understanding of microglia function comes predominantly from rodent studies due to the difficulty of sourcing human tissue and isolating the microglia from these tissues. But there are genetic and functional differences between microglia in mice and humans, so these studies may not accurately represent many human conditions," says lead author Wenxin Ma, a Ph.D. Biologist at the Retinal Neurophysiology Section, National Eye Institute, National Institutes of Health, US.
"To address this concern, researchers have been growing human microglia from human stem cells. We wanted to take this a step further and see if we could transplant human microglia into the mouse retina, to serve as a platform for screening therapeutic drugs as well as explore the potential of microglia transplantation as a therapy itself," adds senior author Wai Wong, Vice President of Retinal Disease, Janssen Research and Development LLC, California, U.S.
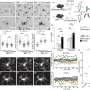
To test their approach, the researchers grew microglia from a type of stem cell called human induced pluripotent stem cells (hiPSCs). They conducted a series of tests to check that their cultivated microglia cells functioned as typical immune cells and then transplanted them into mice retina.
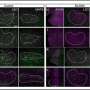
First, they used a drug to eliminate the existing population of microglial cells, and then they transplanted the new, labeled cells in their place. At four and eight months after transplantation, the human microglial cells had migrated into the retina and were well distributed, and behaving as microglia should—responding to chemical signals within the eye. Moreover, the introduction of hiPSC-microglia cells had no negative effects on the surrounding cells of the retina.
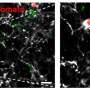
Having shown that these transplanted hiPSC-microglia integrate harmoniously with the existing mouse microglia population, the team tested whether the transplanted cells had a normal immune reaction to retinal cell injury. They found that the cells behaved exactly as the resident mouse microglia—migrating into the subretinal space to be near damaged cells. This left a lower number of human microglia in their original location, but the transplanted microglia quickly replenished themselves, and stopped multiplying once back to their original numbers.
An important role of microglia is to gobble up unhealthy cells in a process called phagocytosis. Not only did the transplanted microglia move into the damaged regions, they also hoovered up the damaged light-receiving cells. This further suggests that the transplanted cells mirrored normal functions of resident microglia in the eye.
One limitation of the study is uncertainty around its claim that the microglia generated from hiPSCs are mature, and therefore comparable to human-derived mature microglia cells. Further work is needed, according to the eLife public review, to claim they are mature human microglia, such as carrying out a comparative analysis with publicly available gene expression data for primary human microglia.
"Understanding microglial cell function is essential for investigating disease mechanisms and identifying accurate targets for treating degenerative retinal diseases," explains senior author Wei Li, a Senior Investigator in the Retinal Neurophysiology Section, National Eye Institute, National Institutes of Health. "Our model provides a new way of studying the functions of human microglia within disease mechanisms and a platform for evaluating the potential therapeutic effects of microglia cell transplants from diverse patient backgrounds."
More information: Wenxin Ma et al, Human iPSC-derived Microglia Cells Integrated into Mouse Retina and Recapitulated Features of Endogenous Microglia Cells, eLife (2023). DOI: 10.7554/eLife.90695.1