This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Scientists take next big step in understanding genetics of schizophrenia
Genetically speaking, we are individuals different from each other because of slight variations in our DNA sequences—so-called genetic variants—some of which have dramatic effects we can see and comprehend, from the color of our eyes to our risk for developing schizophrenia—a debilitating psychiatric condition affecting many millions worldwide.
For several years, scientists have studied the entire genomes of thousands of people—called genome-wide association studies, or GWAS—to find approximately 5,000 genetic variants associated with schizophrenia.
Now, UNC School of Medicine scientists and colleagues are figuring out which of these variants have a causal effect in the development of the schizophrenia. They are finding that some of genetic variants regulate or alter the expression of genes involved in the condition.
Published in the journal Cell Genomics, this research marks a big step forward in our understanding of the genetic basis of schizophrenia.
"Our findings not only provide insights into the intricate regulatory landscape of genes, but also propose a groundbreaking approach to decoding the cumulative effect of genetic variants on gene regulation in individuals with schizophrenia," said senior author Hyejung Won, Ph.D., associate professor of genetics at the UNC School of Medicine. "This comprehension could potentially pave a path for more precise interventions and therapies in the future. Right now, therapeutic options are limited, and some people do not respond to drugs available."
For this study, Won and first authors Jessica McAfee and Sool Lee, both UNC-Chapel Hill graduate students, led a team of researchers from UCLA, Harvard, the University of Michigan, and Human Technopole in Italy to explore the genetic variants already linked to the risk of schizophrenia through GWAS research.
Their goal was to figure out a way to tease apart meaningless variants from those with potential for biological activity important for developing schizophrenia. This isn't easy for a few reasons, one of which is that genetic variants are often inherited together from parents. So, right next to each other could be two genetic variants associated with the condition—one might be important for gene expression that plays a major role in the condition, but the other variant might not have any role in the condition.
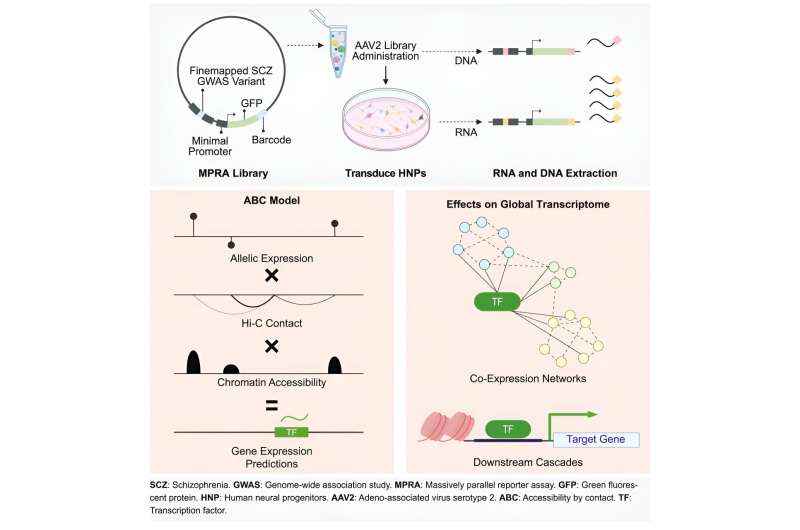
To tackle this problem, the researchers used a special technique called a massively parallel reporter assay (MPRA)—essentially a genetic sequencing technique that can parse which variants trigger gene expression and which ones don't. To use this method, the researchers introduced the 5,000 variants into human brain cells in a dish, cells that are essential for early brain development.
These variants may or may not cause the expression of their downstream gene and genetic barcode. The barcode, a 20bp DNA sequence, is unique to each variant. This is what the group uses to distinguish the variant sequences. The MPRA revealed 439 genetic variations with actual biological effects, meaning they can alter expression of gene.
"Traditionally, scientists have used other epigenetic data, such as transcription factor binding and biochemically defined enhancers, to identify variants with biological effects," Won said.
"However, these conventional methods failed to predict a large portion of variants we identified to have biological effects. Our work points to a wealth of unexplored variants with biological effects."
To understand how these variants work together to influence gene activity, Won and colleagues developed a new model that combines data from MPRA with chromatin architecture of brain cells—that is, the genetic information important for how brain cell DNA is organized. By doing this, the researchers could connect these 439 variants to how genes are turned on or off.
"Schizophrenia is a complex condition that is highly heritable," Won said. "To find these 439 potentially causal variants is a big step, but we still have a lot of work ahead to figure out the complicated genetic architecture that leads an individual to develop this condition. With that information in hand, we could begin to understand the biological mechanism underlying this complex disorder, which may eventually lead to targeted therapies."
More information: Jessica C. McAfee et al, Systematic investigation of allelic regulatory activity of schizophrenia-associated common variants, Cell Genomics (2023). DOI: 10.1016/j.xgen.2023.100404