This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Strep molecule illuminates cancer immune therapies
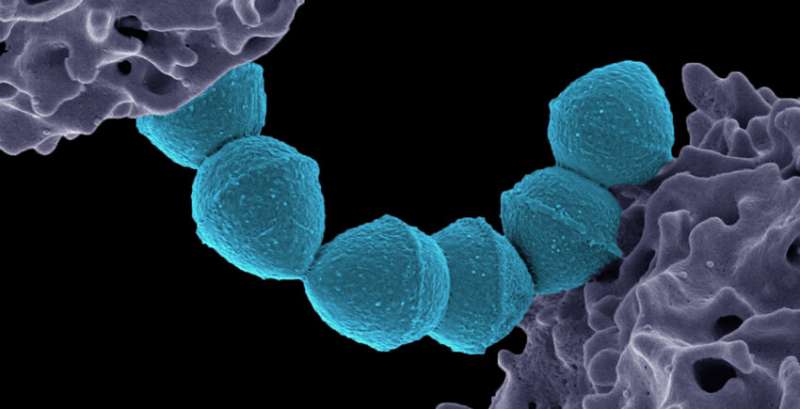
Researchers at Harvard Medical School have discovered that a molecule made by Streptococcus pyogenes—the bacterium that causes strep throat and other infections—could help explain several long-standing medical mysteries, such as why strep sometimes leads to serious immune complications, including rheumatic fever; how the immune system's recognition of the molecule may contribute to diseases like lupus; why one of the first cancer immunotherapies showed promise more than 100 years ago; and how current immune therapies for cancer could be more effective.
The findings also contradict a long-standing belief that the immune system ignores this bacterial molecule and could propel efforts to tame or activate the immune system to treat a range of diseases.
The team, led by the lab of HMS biochemist Jon Clardy, published its findings Sept. 22 in the Journal of the American Chemical Society.
"We were very surprised by the results, but the data were compelling," said Clardy, the Christopher T. Walsh Ph.D. Professor of Biological Chemistry and Molecular Pharmacology in the Blavatnik Institute at HMS.
From Coley's toxins to cardiolipins
A surgeon named Friedrich Fehleisen discovered in 1883 that S. pyogenes caused a rash known to spread across the skin of some people whose cancers then inexplicably disappeared.
Another surgeon, William Coley, then revealed that infecting otherwise untreatable cancer patients with S. pyogenes or other bacterial strains—dubbed "Coley's toxins"—sometimes cured their disease. But many of his peers didn't believe the results, and his methods soon fell out of favor as radiation and chemotherapy hit the stage.
Coley's reputation was restored with the advent of modern cancer immunotherapies that harness the immune system to vanquish tumors, but until now no one had figured out what made his original treatments work.
Clardy and colleagues at HMS homed in on an answer using an immune activation test. The test identifies bacterial molecules that stimulate an immune response in cell cultures derived from mouse bone marrow.
Only one molecule in S. pyogenes did the trick: an unassuming fatty molecule, known as a cardiolipin, in the cell membrane.
The team named it SpCL-1, for the first S. pyogenes cardiolipin.
This is the first time researchers have implicated a bacterial cardiolipin in human immune responses.
"The conventional wisdom is that simple membrane lipids like the bacterial cardiolipins in this paper are not immunogenic," said Clardy. "This result upends that belief."
Rheumatic fever and other autoimmune diseases
Our own cell membranes contain cardiolipins too. In some autoimmune diseases and rheumatic fever, the body, for unclear reasons, makes antibodies that attack these molecules.
The new study provides evidence that such diseases can get kickstarted when the immune system reacts to cardiolipins in invading bacteria. After fighting off the bacteria, the primed antibodies can then turn on the person's own cardiolipins.
"Our work provides an answer to the mystery of why, in some of these poorly understood autoimmune diseases, the body develops an immune response to self-antigens—why it attacks itself," Clardy said.
The insights could help researchers in their efforts to prevent these and related autoimmune disorders.
Bacteria and cancer immunotherapy
On the flip side, the work offers insights into how bacterial molecules can help scientists harness the immune system to treat disease.
The team found that two receptors on the surface of many immune cells, known as toll-like receptors, lock onto S. pyogenes cardiolipins. The cells then recognize the bacteria as invaders and call out for release of an inflammatory molecule known as tumor necrosis factor (TNF) alpha.
The cardiolipin therefore could provide an additional strategy to stimulate an immune response to fight cancer.
Such a strategy would join other efforts that build on the discovery that some patients' cancers trick immune cells into not secreting TNF alpha and other molecules that ought to kill them.
Whether the cardiolipin SpCL-1 itself could become or inspire a successful new cancer immunotherapy remains to be seen, although Coley's toxins provide a promising foundation.
"The historical record provides some reason for optimism," the authors said.
Meanwhile, the findings also could help researchers better understand why cancer immunotherapies don't work in all patients. Studies from HMS and beyond have shown that the gut microbiome influences immunotherapy effectiveness, and bacterial cardiolipins may add a piece to the puzzle.
Clardy's team previously uncovered another simple fatty molecule in a gut bacterium, A. muciniphila, that could explain how that bacterium helps make cells more responsive to cancer immunotherapies.
"There is a lot of interest in why cancer immunotherapy has such a mixed and unpredictable outcome," said Clardy. "It would be a privilege to help answer that question."
More information: Yern-Hyerk Shin et al, Revisiting Coley's Toxins: Immunogenic Cardiolipins from Streptococcus pyogenes, Journal of the American Chemical Society (2023). DOI: 10.1021/jacs.3c07727