This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A possible new treatment for acute myeloid leukemia
New research has identified a novel immunotherapy for acute myeloid leukemia. The study, published in Nature Cancer, describes a T-cell receptor that recognizes a mutation shared between a subgroup of patients with the disease. The results provide hope for new and effective treatment using T cells equipped with the therapeutic T cell receptor, "programmed" to kill the leukemia cells.
Acute myeloid leukemia (AML) is the most frequent form of leukemia in adults. The disease develops very rapidly, and has a very poor prognosis, with an average overall 5-year survival rate of 29% with standard therapy. Immunotherapy has led to significant advances in the treatment of several types of cancer in later years. However, there is currently no approved immunotherapy for AML except for stem cell transplantation, which is a treatment with potentially life-threatening side effects.
The research group of Professor Johanna Olweus at the University of Oslo (UiO) and Oslo University Hospital (OUS), in collaboration with Professor Sten Eirik Jacobsen's and researcher Petter Woll's research groups at Karolinska Institutet (KI) in Stockholm, has now identified a possible target for the treatment of AML.
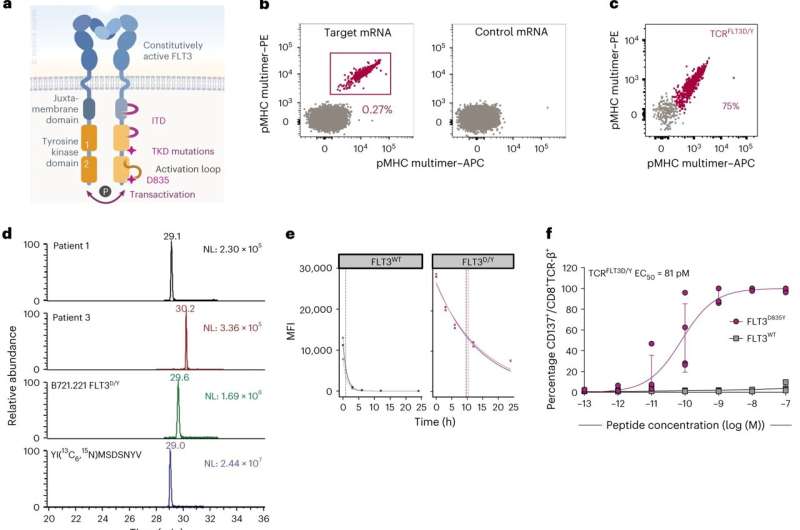
The researchers have provided important proof-of-concept that T-cell receptor (TCR) T cells targeted to mutations shared between patients, can be an attractive therapeutic option in AML. "This provides hope that we can develop a new and effective treatment for acute myeloid leukemia, with likely relevance also for other cancer types," says Professor Johanna Olweus.
Recent research has provided hope that immunotherapy can be targeted to mutations, as mutations are specific for cancer cells and are a necessary part of cancer development. However, the results have been discouraging so far.
"Almost all mutations are unique to the individual cancer tumor and patient, and targeted treatment must therefore be tailored to each individual patient. We also know that many mutations exist only in some cancer cells, which allows other cancer cells to escape treatment. In addition, only few mutations are recognized by the immune cells, Professor Olweus explains.
However, certain rare mutations are shared between subgroups of patients. The authors of the study were interested in exploring the potential of utilizing such mutations as targets for immunotherapy. One gene that often is mutated in AML is FLT3, which can contribute to disease acceleration. Using technology developed in Olweus' group, postdoc Eirini Giannakopoulou and colleagues identified a T-cell receptor that recognizes the mutation. Giannakopoulou, first author of the study, describes the discovery as "finding a needle in a haystack."
The researchers' then demonstrated that the T-cell receptor was safe. Next, they showed that the T-cell receptor effectively eliminated leukemia cells in multiple disease-relevant models. Such data for TCR-T cells or CAR-T cells are rare in the field.
"Here, the expertise on AML models in the groups of Woll and Jacobsen was crucial. The animal studies in advanced models where leukemia cells from patients were transplanted into mice, were conducted at KI in collaboration between the groups," Olweus says.
The treatment proved to be highly effective in eliminating mutated leukemia cells in just two weeks. "An important finding was that we were able to show that TCR-T cells could also kill cells with characteristics of leukemia stem cells," says doctoral candidate Madeleine Lehander, second author and a central contributor to the study, and supervised by Woll and Jacobsen.
Numerous members of the UiO/OUS group and the KI groups are co-authors on the article, which Olweus describes as "a fantastic collaboration that has spanned many years, where the groups complement each other's expertise perfectly." This is the second major collaboration project between the groups—the first was published in Nature Biotechnology in 2022.
More information: Giannakopoulou, E. et al, A T cell receptor targeting a recurrent driver mutation in FLT3 mediates elimination of primary human acute myeloid leukemia in vivo, Nature Cancer (2023). DOI: 10.1038/s43018-023-00642-8 www.nature.com/articles/s43018-023-00642-8