This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Drug screen points toward novel diabetes treatments
A drug currently in clinical trials as a cancer therapy can also stimulate pancreatic beta cells to secrete insulin, revealing a previously unknown mechanism for insulin regulation in type 2 diabetes, according to a new study by Weill Cornell Medicine investigators. The preclinical discovery, reported in Nature Chemical Biology, provides a new chemical tool for probing the biology of diabetes, and could point the way toward better treatments for the disease.
"We have known about insulin for a century, but when it comes to the major mechanisms controlling insulin secretion, there are still a lot of things which are unknown," said senior author Dr. Shuibing Chen, director of the Center for Genomic Health and the Kilts Family Professor of Surgery at Weill Cornell Medicine.
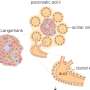
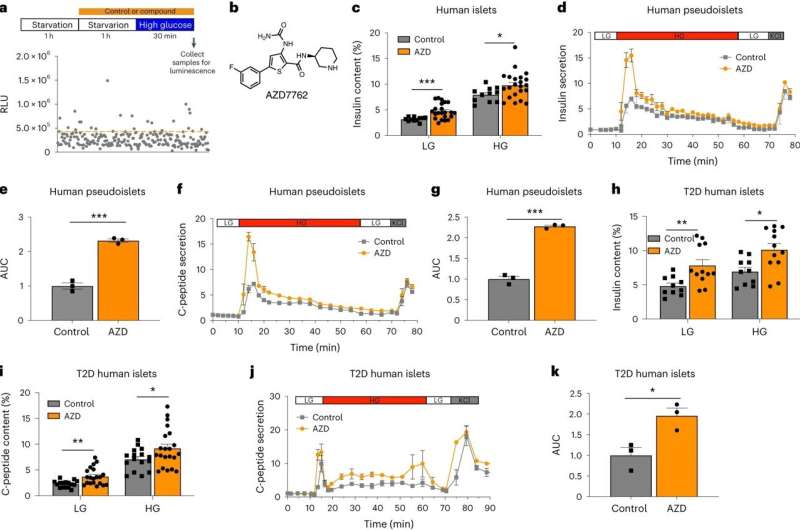
To search for new insulin-regulating mechanisms, Dr. Chen and her colleagues, including first author Dr. Angie Chi Nok Chong, a postdoctoral associate in Dr. Chen's lab, began by testing a library of drugs on cultures of mouse pancreatic beta cells, looking for compounds that stimulated insulin secretion in response to glucose.
"Instead of running a large-scale drug screening, we decided to do a focused library," said Dr. Chen who is also a member of the Hartman Institute for Therapeutic Organ Regeneration at Weill Cornell Medicine. "So, we had candidates that we knew might be involved in certain important biological processes."
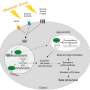
The screen identified three compounds that promoted insulin secretion, two of which targeted known insulin-regulating mechanisms in pancreatic beta cells. A third, however, targeted a protein called CHEK2, which is often mutated in cancer but hadn't previously been associated with glucose metabolism.
With collaborators at two other institutions, the researchers confirmed that the compound increases glucose-mediated insulin secretion in multiple tests on human and non-human pancreatic cells grown in the laboratory, and various mouse models of type 2 diabetes. "That makes the data very exciting, because it's really consistent from mouse to human islets in vitro, as well as both mouse and nonhuman primate in vivo," said Dr. Chen.
Probing the mechanism more closely, the team uncovered a previously unknown insulin secretion-regulating molecular pathway that operates in multiple mammalian species. Dr. Chen says the compound they identified in their initial screen, called AZD7762, promises to be a useful tool for exploring other aspects of insulin regulation.
"We not only identified CHEK2 as a potential drug target; we can also use AZD7762 as a chemical probe to find new mechanisms for insulin secretion, and find new targets in the future," she said.
While AZD7762 is currently in clinical trials as a cancer therapy, it's unlikely to be directly adapted to a new diabetes treatment. "The safety requirement for cancer therapy and diabetes therapy is completely different," Dr. Chen said, adding that the side effects of the cancer drug would likely make it unsuitable for long-term use in a chronic condition such as type 2 diabetes. However, compounds that affect CHEK2 only in pancreatic beta cells could prove very effective.
"If we can target delivery of the drugs to beta cells without affecting other cells, that could be an interesting approach to probe," said Dr. Chen.
The project has also laid the groundwork for future drug development. "We built up the foundation for the chemical screening and testing in animal and cell models," Dr. Chen said. "In the future if we find anything interesting, it can be immediately tested in this pipeline."
More information: Chong, A.C.N. et al, Checkpoint kinase 2 controls insulin secretion and glucose homeostasis, Nature Chemical Biology (2023). DOI: 10.1038/s41589-023-01466-4. www.nature.com/articles/s41589-023-01466-4