This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study links gene network and pancreatic beta cell defects to type 2 diabetes

A comprehensive study that integrates multiple analytic approaches has linked a regulatory gene network and functional defects in insulin-producing pancreatic beta cells to type 2 diabetes. The study, published Nov. 8 in the journal Nature, lays the foundation for identifying additional early disease-driving events for type 2 diabetes, and it also provides a template for identifying regulatory networks that drive other diseases.
Type 2 diabetes affects almost 35 million people in the United States, increasing the risk of death and causing serious health complications including blindness, kidney failure, heart disease and stroke, according to the Centers for Disease Control and Prevention.
Genome-wide association studies (GWAS) have linked hundreds of sites in the genome to increased risk of type 2 diabetes, but 90% of the sites are located in non-coding rather than protein-coding regions of DNA.
"How this genetic variation at the population level relates to molecular changes in gene expression, tissue architecture and cellular physiology in type 2 diabetes is not well understood," said Marcela Brissova, Ph.D., research professor of Medicine at Vanderbilt University Medical Center and lead co-corresponding author of the new study. Jack Walker, MD, Ph.D., who was a student in Vanderbilt's Medical Scientist Training Program, and Diane Saunders, Ph.D., research assistant professor of Medicine at VUMC, are co-first authors of the paper.
Although processes that contribute to type 2 diabetes have been studied in rodent models, human islets—the mini-organs that house beta cells along with a number of other cell types—differ from rodent islets in multiple ways.
"There is a need for studies to define the mechanisms that initiate and sustain islet dysfunction in primary human islets, which is where we focused our efforts," said Alvin C. Powers, MD, director of the Vanderbilt Diabetes Research and Training Center, Joe C. Davis Professor of Biomedical Science and a co-corresponding author of the study.
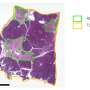
Critical aspects of the group's approach include the use of the Vanderbilt Pancreas Biorepository that has been assembled over the last 10 years and the study of pancreatic tissue and isolated islets from the same donors.
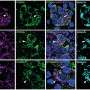
The Vanderbilt team used an integrated, multimodal approach to study pancreas and islets from donors with early-stage type 2 diabetes and controls. They analyzed islet function ex vivo and in vivo (in a mouse model), performed comprehensive transcriptional (gene expression) analysis using RNA-sequencing, and assessed islet cellular architecture using multiplex imaging.
"The relative contributions of impaired beta cell function and reduced beta cell mass have long been debated in type 2 diabetes," Saunders said. "Our data indicate that beta cell loss is not a major component in disease pathogenesis in early-stage type 2 diabetes."
Working with co-corresponding author Stephen Parker, Ph.D., and his colleagues in the Department of Computational Medicine & Bioinformatics at the University of Michigan and adding the expertise of Jennifer Below, Ph.D., and Hung-Hsin Chen, Ph.D., in the Division of Genetic Medicine at VUMC, the interdisciplinary team connected laboratory findings from single cells to population-scale genetics.
The researchers also performed network analysis to identify gene "modules" that linked beta cell transcriptional profiles with beta cell functional parameters, donor traits and GWAS variants.
The team found that the transcription factor RFX6 is a highly connected hub factor and is reduced in beta cells from individuals with type 2 diabetes.
In further studies of RFX6 and its regulatory network, the researchers used an in vitro human pseudoislet model to show that disruption of RFX6 in beta cells led to reduced insulin secretion and altered chromatin architecture at regions enriched for type 2 diabetes GWAS signals. Using phenotype and genotype data from the UK Biobank across nearly one-half million people of European ancestry, they found that predicted decreased islet expression of RFX6 was causally associated with type 2 diabetes.
"Our integrated, multimodal studies identify beta cell dysfunction arising within the beta cell—including from an RFX6-mediated network—as a key event in early-stage type 2 diabetes pathogenesis," Brissova said. "Precisely what underlies the initial RFX6 dysregulation and whether it can be targeted to prevent or reverse early-stage molecular and functional defects in the beta cell will be important areas of further investigation."
More information: Marcela Brissova, Genetic risk converges on regulatory networks mediating early type 2 diabetes, Nature (2023). DOI: 10.1038/s41586-023-06693-2. www.nature.com/articles/s41586-023-06693-2