This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New age-related macular degeneration study uses light-sensitive retinal organoids
A partnership between ophthalmology researchers at the University of Colorado School of Medicine and Johns Hopkins University expands the understanding of how oxidative stress contributes to the development of choroidal neovascularization (CNV) in patients with age-related macular degeneration (AMD).
To study the roles oxidative stress, a condition in which the body lacks antioxidants, and hypoxia play in the progression of AMD, Johns Hopkins University researchers turned to CellSight, the ocular stem cell and regeneration research program in the CU Department of Ophthalmology, for tools that allow researchers to explore specific conditions relevant to AMD.
Using human-induced pluripotent stem cells, a type of stem cells that are generated or induced from cells obtained from an adult person's skin or blood, CellSight investigators can recreate human retinal tissue in the lab.
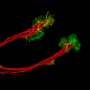
"We generate retinal organoids, which can be described as mini retinas in a petri dish, that mimic the cellular organization of the human retina and are capable of responding to light," explains CellSight researcher Miguel Flores-Bellver, Ph.D., assistant professor of ophthalmology.
"We also have another tool, retinal pigment epithelium tissue, also derived from stem cells. By subjecting these retinal organoids and the retinal pigment epithelium tissues to oxidative stress and hypoxia, we can mimic pathological conditions that promote the development of AMD and contribute to the research our colleagues at Johns Hopkins are conducting."
The research, published in Proceedings of the National Academy of Sciences , marks a step forward in better understanding AMD, a leading cause of vision impairment across the world, and highlights the vital role retinal organoids can play in finding a treatment for millions of people who are diagnosed with the debilitating disease.
Impact of retinal organoids on research
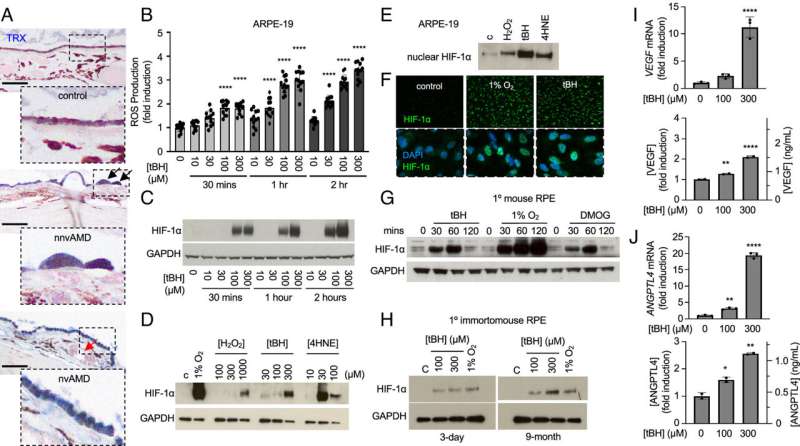
The mini human retina system developed by CellSight researchers in 2014 quickly became a landmark in the field of stem cells and modeling retinal diseases. The most recent endeavor with Johns Hopkins researchers allowed the CU faculty members to showcase once again the range of applications and impact that their retinal organoid technology has in ophthalmology research, by controlling the conditions the organoids are in. In this case, that meant limiting the amount of oxygen to mimic a hypoxic condition.
"It's a very tunable system," Flores-Bellver says of the process used to grow the organoids. "By inducing hypoxia, it helped trigger mechanisms that allowed us to identify possible ways to prevent the development or the progression of the disease."
The ability to mimic the conditions associated with AMD is significantly helpful to researchers, adds CellSight director Valeria Canto-Soler, Ph.D., associate professor of ophthalmology and Doni Solich Family Chair in Ocular Stem Cell Research.
"It's quite meaningful to be able to tangibly use these systems and confirm that they have the capacity to mimic mechanisms that are involved in human diseases," she says. "It also gives us some insight on other mechanisms that could eventually be manipulated to discover further treatments for AMD."
A perfect partnership
Canto-Soler and Flores-Bellver say the work with Johns Hopkins is a natural fit. The study concludes that "a careful balance of hypoxia-inducible factor levels must be maintained to prevent vision loss in the eyes of patients with AMD" and that modulation of hypoxia-inducible factor may be an effective therapeutic approach for the treatment or prevention of AMD.
CellSight members Silvia Aparicio-Domingo and Timothy Domashevich also contributed to the study. The team hopes the work will lead to more breakthroughs for AMD researchers and, ultimately, the patients who live with the disease.
"We need to understand more about AMD because it is the leading cause of blindness in the developed world, and projections show that even more people will have the disease in the next decade," Flores-Bellver says. "Our goal at CellSight is to keep uncovering the mechanisms that contribute to the disease's progression so that one day we can stop it."
"It's exciting," Canto-Soler says of the research. "The possibility of finding cures for patients is what motivates us to get to work every day."
More information: Savalan Babapoor-Farrokhran et al, Pathologic vs. protective roles of hypoxia-inducible factor 1 in RPE and photoreceptors in wet vs. dry age-related macular degeneration, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2302845120