This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
ATR inhibition using gartisertib in patient-derived glioblastoma cell lines
A new research paper titled "ATR inhibition using gartisertib enhances cell death and synergises with temozolomide and radiation in patient-derived glioblastoma cell lines" has been published in Oncotarget.
Glioblastoma cells can restrict the DNA-damaging effects of temozolomide (TMZ) and radiation therapy (RT) using the DNA damage response (DDR) mechanism which activates cell cycle arrest and DNA repair pathways. Ataxia-telangiectasia and Rad3-Related protein (ATR) plays a pivotal role in the recognition of DNA damage induced by chemotherapy and radiation causing downstream DDR activation.
In this new study, researchers Mathew Lozinski, Nikola A. Bowden, Moira C. Graves, Michael Fay, Bryan W. Day, Brett W. Stringer, and Paul A. Tooney from University of Newcastle, Hunter Medical Research Institute, GenesisCare, QIMR Berghofer Medical Research Institute, and Griffith University investigated the activity of the ATR inhibitor gartisertib alone, and in combination with TMZ and/or RT, in multiple patient-derived glioblastoma cell lines.
"Using a panel of 12 patient-derived glioblastoma cell lines, we investigated the chemo- and radio-sensitizing effect of gartisertib, a potent and selective inhibitor of ATR that was explored in a phase 1 clinical trial for patients with advanced solid tumors (NCT02278250)," the researchers explain.
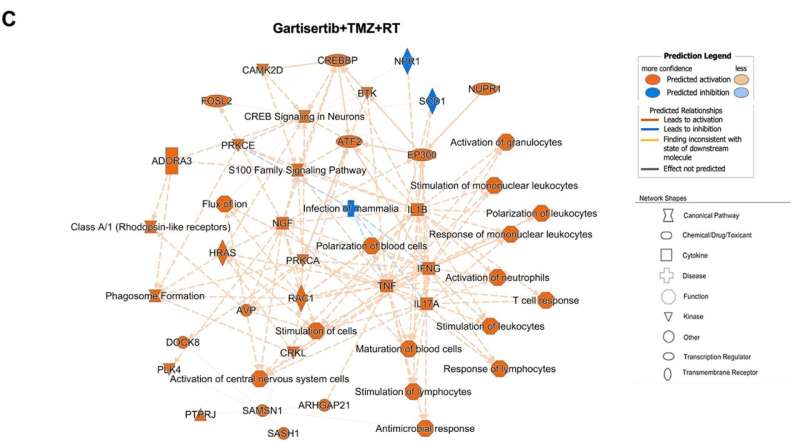
The team showed that gartisertib alone potently reduced the cell viability of glioblastoma cell lines, where sensitivity was associated with the frequency of DDR mutations and higher expression of the G2 cell cycle pathway. ATR inhibition significantly enhanced cell death in combination with TMZ and RT and was shown to have higher synergy than TMZ+RT treatment. MGMT promoter unmethylated and TMZ+RT resistant glioblastoma cells were also more sensitive to gartisertib. Analysis of gene expression from gartisertib treated glioblastoma cells identified the upregulation of innate immune-related pathways.
"Overall, this study identifies ATR inhibition as a strategy to enhance the DNA-damaging ability of glioblastoma standard treatment, while providing preliminary evidence that ATR inhibition induces an innate immune gene signature that warrants further investigation," the researchers conclude.
More information: Mathew Lozinski et al, ATR inhibition using gartisertib enhances cell death and synergises with temozolomide and radiation in patient-derived glioblastoma cell lines, Oncotarget (2024). DOI: 10.18632/oncotarget.28551