This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers create first model of the most common genetic cause of childhood interstitial lung disease
Childhood interstitial lung disease (chILD) is a group of rare, genetic lung disorders affecting infants and children who typically present with a range of symptoms, such as shortness of breath, rapid breathing and coughing. While many causal genes have been identified for chILD, understanding its pathogenesis has been impeded by an inability to easily access primary human cells from affected children and limited methods to maintain these cells from explanted tissue of affected children.
In a new study from the Center for Regenerative Medicine (CReM) of Boston University and Boston Medical Center with collaborators at Washington University School of Medicine, researchers demonstrate how they can now study the most common genetic cause of this devastating pediatric lung disease using stem cells generated from each patient.
These findings appear online in the Journal of Clinical Investigation.
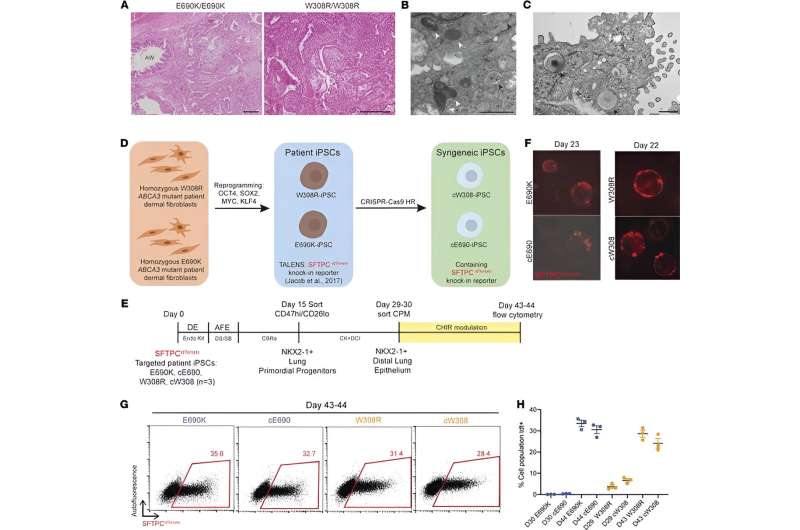
The cells called induced pluripotent stem cells (iPSCs) were engineered in culture dishes using skin cells obtained from children with chILD who also carry mutations in a gene called, ABCA3, the most common genetic cause of the disease. The iPSCs in the culture dish were then changed into the lung cell type responsible for causing the disease, called alveolar epithelial type 2 cells.
The researchers saw several quantifiable differences between the diseased cells made from affected children compared to their cells that had been gene edited to correct the ABCA3 mutation. These results allowed the researchers to better understand the disease, opening the door for therapeutic discovery.
"Development of therapeutic alternatives has been challenging due to an inability to access and culture cells that contain mutations in a gene from patients with chILD. In the present study, we overcame these hurdles by developing and extensively characterizing an in-vitro disease model," explained first author Yuliang (Leon) Sun, MD, Ph.D., who received his dual degree from Boston University in 2022 and whose dissertation was the genesis for this research.
According to the researchers, ABCA3 mutant cells struggled to produce surfactants, a hallmark of alveolar epithelial type 2 cells that help the lung maintain its shape.
"ABCA3 mutant cells also had smaller lamellar bodies, which are organelles specific to alveolar epithelial type 2 cells in which surfactants are made, compared to the health cells. Additionally, we found ABCA3 mutant cells showed higher amounts of inflammation than their healthy counterparts," said co-author Erin Hennessey, a Ph.D. student at the school currently leading the project's continuation.
The researchers believe this study is a critical step forward to understanding and developing a cure for chlLD and highlights the utility of pluripotent stem cells for disease modeling. The study uses methods patented by the CReM to produce lung cells from iPSCs, which can be made from a skin or blood sample from anyone.
The iPSCs banked in the CReM as part of their national lung disease-specific iPSC repository represent an Open Source resource that is widely shared with the global research community to help find cures for lung diseases, including chILD, for which there are inadequate current treatments.
"We hope the growing number of available iPSCs made from children with chILD in our stem cell repository will help our colleagues across the world combat this awful disease," said senior author, Darrell N. Kotton, MD, who is the David C. Seldin Professor of Medicine at Boston University Chobanian and Avedisian School of Medicine and Director of the CReM.
More information: Yuliang L. Sun et al, Human pluripotent stem cell modeling of alveolar type 2 cell dysfunction caused by ABCA3 mutations, Journal of Clinical Investigation (2024). DOI: 10.1172/JCI164274