This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers conduct in-depth characterization of a major gene involved in neurodegenerative diseases
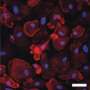
Within our brain cells, there exists a highway system of tube-like structures called microtubules. They give the cell structure and help transport nutrients and other important substances from one part of the nerve cells to another. Tau is an important protein that normally binds to these microtubules.
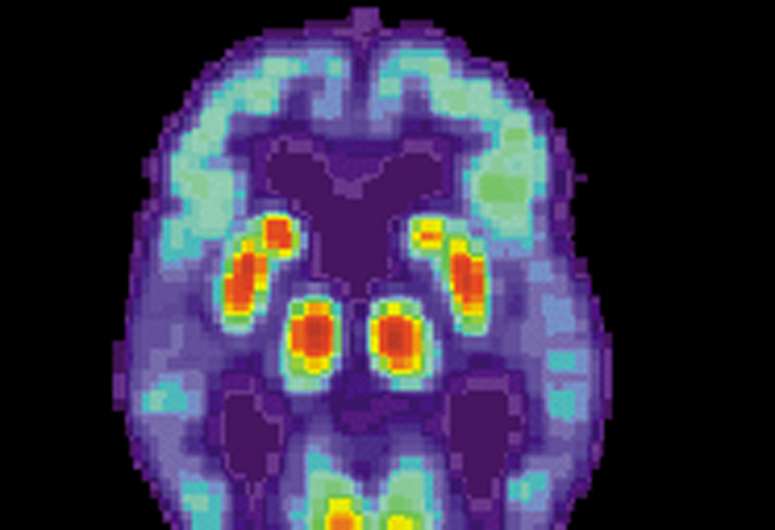
Abnormalities in tau are associated with many brain diseases, including Alzheimer's disease. In Alzheimer's disease, the tau proteins change shape, sticking to each other in tangles and no longer binding to microtubules. This aberrant tau disrupts how neurons and other brain cells function and communicate with each other, causing some of the symptoms of Alzheimer's disease.
Several studies indicate that reducing tau levels prevents the classical damage induced by aberrant tau. Nick Cochran, Ph.D., faculty investigator at HudsonAlpha Institute for Biotechnology, and his lab work to understand tau and its expression at a deeper level in hopes of discovering what goes amiss and how to best prevent or reverse it.
"The more we learn about the human genome, the more genetic elements emerge that are involved in healthy and diseased states," says Cochran. "Coding genetic variation is no longer the sole focus of understanding genetics' role in disease pathology.
"Stretches of DNA called cis-regulatory elements, as well as the transcription factors and other elements that are all, in aggregate, responsible for turning genes on and off, have major implications for human disease. Diving into the regulation of genes will lead to a more holistic picture of how variation in our genomes leads to disease risk, allowing us to better understand and target the problem involved in these diseases."
In a new study published in The American Journal of Human Genetics, Cochran and his team, as well as the Myers lab at HudsonAlpha, used many genomic technologies to better understand MAPT expression and identify new regulatory regions that could one day be the target of therapeutics.
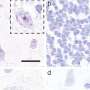
"This represents a very in-depth analysis of MAPT expression," says Bri Rogers, Ph.D., postdoctoral fellow and first author of the study. "We began by coupling gene expression and DNA accessibility data at the single-cell level with cultured neurons and brain tissue. Once we identified interesting potential regulatory elements, we used many orthogonal approaches to confirm they regulated MAPT expression."
Through these exhaustive studies, the team identified 97 candidate regulatory elements that control MAPT expression. They focused predominantly on non-coding cis-regulatory elements that regulate MAPT, some from nearby and others from far away. Using CRISPRi, researchers confirmed whether the nominated elements regulate MAPT.
"These findings are exciting because many elements we found are so far away from MAPT that variants within these regulatory regions would not have been associated with MAPT previously," says Rogers. "Linking these regulatory elements to MAPT really helped us to understand the potential consequences of variants in these regions."
The team also identified interesting rare variants in some MAPT regulatory elements, but these rare variants seem to act in a protective rather than deleterious way. The variants are present in individuals who do not have Alzheimer's and are depleted in those with Alzheimer's disease. Cochran and his team hypothesize that these variants are damaging to the enhancer element, thus reducing MAPT expression and protecting against neurodegenerative disease.
"It's important to note that rare genetic variants can be both deleterious and protective when it comes to their effect on disease risk. While it is still early days for analysis of rare non-coding variant effects on disease risk, it is likely that this is the first of many discoveries where this category of genetic variation will prove to be important," says Cochran.
This study provides a better understanding of how genetic variants around MAPT contribute to disease risk. By identifying transcription factors that bind to the regulatory regions, future studies could reveal new therapeutic targets for Alzheimer's disease and other tauopathies.
More information: Brianne B. Rogers et al, Neuronal MAPT expression is mediated by long-range interactions with cis-regulatory elements, American Journal of Human Genetics (2024). DOI: 10.1016/j.ajhg.2023.12.015. www.cell.com/ajhg/fulltext/S0002-9297(23)00447-0