This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Study: In patients with long COVID, immune cells don't follow the rules
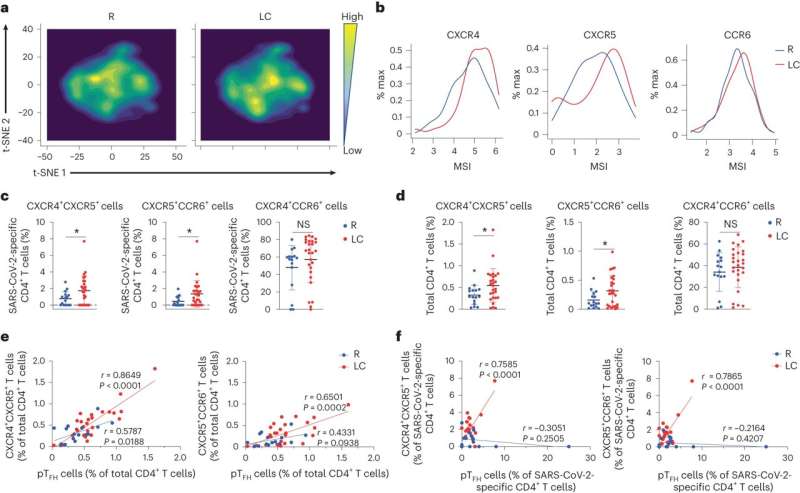
People with long COVID have dysfunctional immune cells that show signs of chronic inflammation and faulty movement into organs, among other unusual activity, according to a new study by scientists at Gladstone Institutes and UC San Francisco (UCSF).
The team analyzed immune cells and hundreds of different immune molecules in the blood of 43 people with and without long COVID. They delved particularly deep into the characteristics of each person's T cells—immune cells that help fight viral infections but can also trigger chronic inflammatory diseases.
Their findings, which appear in Nature Immunology, support the hypothesis that long COVID may involve a low-level viral persistence. The study also reveals a mismatch between the activity of T cells and other components of the immune system in people with long-term COVID-19.
"Our results are an essential first step to understanding what's going on with T cells in long COVID," says senior author Nadia Roan, Ph.D., a senior investigator at Gladstone and a professor at UCSF. "This paves a path toward answering ongoing questions about the different types of long COVID, the mechanisms that cause it, and how to treat and prevent it."
A pristine group to study
Long COVID, also known in the medical community as "post-acute sequelae of COVID," or PASC, is broadly defined as symptoms that continue or emerge after an initial infection with the SARS-CoV-2 virus.
The trajectory of long COVID can vary drastically between individuals; some have initial symptoms of COVID that never disappear, some have symptoms that come and go, and others have new symptoms that appear weeks or months after their viral infection. In addition, vaccination status and subsequent infections can impact a person's long COVID risk and disease progression.
"This is a very heterogeneous condition," Roan says. "There's a diverse mix of long COVID cases, making it difficult to work out what's happening. That's why it was so important to eliminate some of this variability. We analyzed and compared a set of pristine samples not complicated by the effects of vaccination or re-infection, which can affect T-cell and other immune responses."
Roan's group teamed up with researchers at UCSF—including infectious disease experts Michael Peluso, MD, and Timothy Henrich, MD—who are part of a multidisciplinary team running an observational COVID study called LIINC, short for Long-term Impact of Infection With Novel Coronavirus.
The study follows a cohort of people who were infected once with COVID in 202 and who weren't vaccinated or reinfected during the next eight months. Those who consistently had symptoms during the entire study period were classified as having long-term, COVID, while those who had no symptoms following their initial infection were classified as the control group.
To study the blood of participants collected eight months post-COVID infection, the team used six different technologies, including one they'd previously implemented to deeply interrogate the function of T cells in the context of infection by human immunodeficiency virus (HIV). The technique, called CyTOF, measures levels of different molecules on the surface of or within T cells.
Several key markers of long COVID
While the overall number of T cells and the number of T cells that react specifically with the SARS-CoV-2 virus were similar between people with long COVID and those that recovered without lingering symptoms, the researchers pinpointed several significant differences. Notably, a subset of T cells known as CD4 T cells, which are responsible for the overall coordination of immune responses, were in a more inflammatory state in people with long COVID.
"Not every person with long COVID had these pro-inflammatory cells, but we only saw them in the long COVID group," says Kailin Yin, Ph.D., postdoctoral fellow in the Roan lab and co-first author of the study. "It underscores the idea that there isn't just one uniform thing that characterizes all individuals with long COVID."
In a different subset of T cells known as CD8 T cells, which normally kill cells that are infected by viruses or bacteria, the researchers observed signs of exhaustion preferentially in people with long COVID. These signs, interestingly, were observed only in T cells that recognize the SARS-CoV-2 virus, not in the broader population of CD8 T cells.
"Such exhaustion is typically seen in chronic viral infections such as HIV, and means the T cell branch of the immune system stops responding to a virus and no longer kills infected cells," says Peluso, assistant professor in the UCSF Department of Medicine and co-first author. "This finding fits with some hypotheses that long COVID, or at least some cases of it, are caused by persistent infections by the SARS-CoV-2 virus."
The team also found an unusually high number of "tissue-homing" T cells, which are T cells that are prone to migrating to tissues throughout the body. This was observed not only by CyTOF but also by two other technologies, including one that monitors individual cells for thousands of different proteins they're capable of producing.
"This was really interesting because in other studies we're carrying out in mice, we also see high levels of tissue-homing receptors being associated with behavioral changes after recovery from SARS-CoV-2 infection," Roan says. "In this current study, we don't look at specific tissues, but our results indirectly suggest that in long COVID, something is happening within tissues, recruiting T cells to migrate there."
Finally, the researchers showed that in people with long-term COVID-19, levels of antibodies against SARS-CoV-2 are unusually high, and they don't synchronize as they usually do with levels of T cells that fight the virus.
"This finding points to the notion that during long COVID, you have a breakdown in coordination between different arms of the immune system," says Henrich, a UCSF Department of Medicine professor.
It's important to note that the new study wasn't designed to test any potential treatments for long COVID or to gauge the feasibility of using T-cell markers as a diagnostic. But, Roan says, it does point to new avenues to investigate along those lines. Her team is already planning future experiments on T cells found within specific tissues of people with long COVID and will be looking at how anti-viral and anti-inflammatory drugs could change the characteristics of T cells associated with the disease.
"In the long run, testing interventions will be critical," Roan says. "With a lot of these associations related to long COVID, we don't know what's the chicken and what's the egg until we test treatments."
More information: Kailin Yin et al, Long COVID manifests with T cell dysregulation, inflammation and an uncoordinated adaptive immune response to SARS-CoV-2, Nature Immunology (2024). DOI: 10.1038/s41590-023-01724-6


















