This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Uncovering the link to combating muscle atrophy caused by aging and immobility
The loss of muscle mass, or muscle atrophy, is a relatively common condition in today's aging and increasingly sedentary societies. While the disuse of muscles is the most frequent catalyst for muscle atrophy, there are several other possible causes, including chronic diseases, injury, and exposure to low-gravity environments, such as spaceships. Despite being a prevalent condition, its underlying mechanisms are complex and not entirely understood.
Scientists have shown that mitochondria play essential roles during muscle development, regeneration, and maintenance. In particular, muscle stem cells and differentiated muscle fibers (myofibers) need a lot of energy to become fully mature and functional. Thus, problems with mitochondria can immediately translate to muscle diseases, including muscle atrophy.
In a recent study, a team of researchers, including Junior Associate Professor Takahiko Sato from Fujita Health University, Japan, revealed the close relationship that exists between muscle atrophy, development, and regeneration and the tethering of mitochondria to the endoplasmic reticulum (ER). Their findings were recently published in eLife.
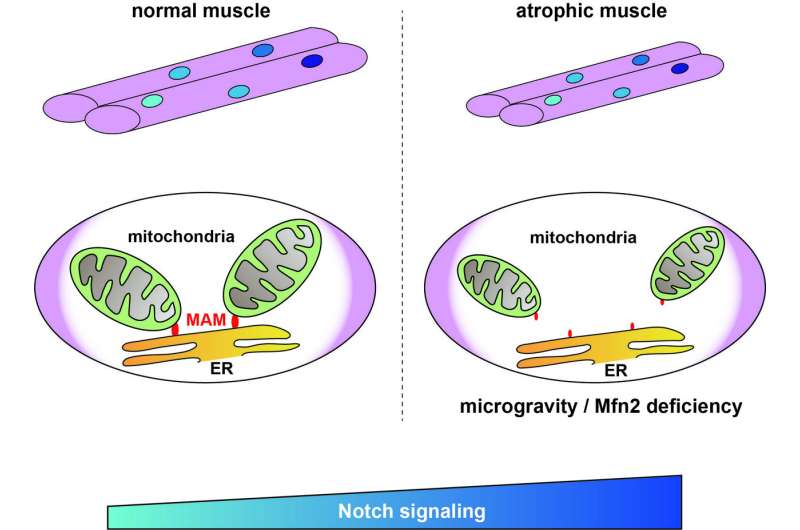
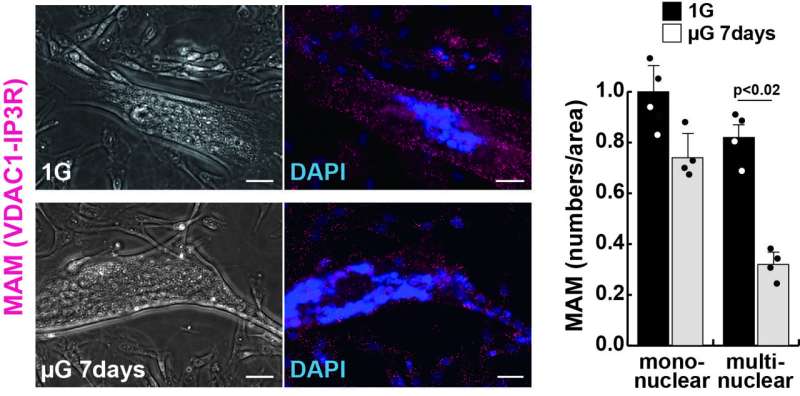
In healthy cells, there are regions in the ER called mitochondria-associated membranes (MAMs) that can be reversibly tethered to the mitochondria. This anchoring serves many functions, such as calcium homeostasis (balance) and regulation of metabolism and mitochondrial morphology. However, whether and how MAMs are involved in the context of muscle atrophy is unclear.
Motivated by this knowledge gap, the research team conducted a variety of experiments involving 'Mitofusin2' (MFN2), a protein that is necessary for mitochondria tethering in MAMs. Skeletal muscle cells cultured in a microgravity environment, known to result in muscle atrophy, exhibited a sharp reduction in the number of MAMs, as well as lower levels of MFN2, alongside typical symptoms of muscle atrophy.
Likewise, human cells with a mutated MFN2 gene exhibited similar traits, as well as abnormalities in mitochondrial fission and lower energy (or ATP) production, indicating problems in the generation of 'cellular energy'.
A closer look at the gene expression profiles in these atrophied muscle cells showed that they all exhibited an upregulation in the Notch signaling pathway. This pathway is essential for cell communication and regulates multiple cell processes, including cell proliferation, differentiation, and programmed death.
Using the gamma-secretase inhibitor DAPT, a known suppressor of Notch signaling, the researchers managed to revert the effects of MFN2 deficiency and partially restore mitochondrial morphology and function, as well as the number of MAMs.
The team further investigated the relationship between MAMs, MFN2, and Notch signaling in muscle atrophy in mice. They analyzed how MFN2 deficiency induced in muscle tissue and the inhibition of Notch signaling affected muscle regeneration after either repeated injury or transplantation of muscle stem cells.
The results were consistent with the in vitro experiments, as Dr. Sato remarks, "Our tests demonstrate that the restoration of ER-mitochondria contacts through the regulation of Notch signaling is sufficient to partially alleviate the bioenergetic defects in atrophied muscle cells. This suggests that treatment with gamma-secretase inhibitors may be a viable therapeutic option in pathological conditions in which MFN2 is involved."
In other words, the findings may reveal new avenues towards the treatment of skeletal muscle atrophy caused by mitochondrial abnormalities.
Further research efforts will be needed to get a more comprehensive understanding of how the complex orchestration of mitochondria and the associated signaling pathways are related to the loss of muscle mass. With eyes on the future, Dr. Sato remains optimistic, as he concludes, "In the future, we may be able to develop new therapeutic treatments that prevent or ameliorate muscle atrophy caused by aging and immobility."
More information: Yurika Ito et al, The reciprocal regulation between mitochondrial-associated membranes and Notch signaling in skeletal muscle atrophy, eLife (2023). DOI: 10.7554/eLife.89381.3