This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Review discusses metabolic reprogramming of T cells
When foreign antigens trigger an immune response, T cells respond by proliferating and differentiating into two groups—effector and memory cells. Epigenetic and transcriptional pathways mediate this response, but the cells also undergo metabolic reprogramming to meet the dynamic biosynthetic demands of proliferation and differentiation.
Cancer and chronic viral infections cause persistent T cell activation to the point of antigen-specific T cell exhaustion, and these exhausted cells are defined by impaired proliferation, effector function, and capacity for self-renewal.
Exhausted T cells have idiosyncratic metabolic demands and are plagued by ineffective mitochondria and glycolytic cycles. Improving T cell fitness through targeted interventions can serve as a promising approach for restoring weakened immune responses.
Now, in a new study, Professor Bo Huang from the Department of Immunology & National Key Laboratory of Medical Molecular Biology, Chinese Academy of Medical Sciences & Peking Union Medical College, China, and his team, reviewed the latest literature on how metabolism influences the fate of T cells.
"In this review, we aimed to elucidate the recent advancements in our understanding of the metabolic changes that occur during effector and memory T cell differentiation in response to pathogen infections and tumorigenesis. Additionally, we focused on the metabolic adaptations exhibited by exhausted T cells when subjected to nutrient deprivation within the tumor microenvironment (TME). Furthermore, we explored how extracellular metabolic stress in the TME influences the metabolic and epigenetic mechanisms that contribute to T cell exhaustion differentiation," says Prof. Huang, while talking about this review.
The article was published in the Chinese Medical Journal on December 12, 2023.
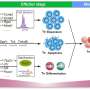
The authors first elaborate on memory T cell formation, describing the sequential stages: T cell activation, expansion, contraction, and the establishment of memory T cells. This involves orchestrated epigenetic changes and transcriptional regulation. After antigen recognition and activation, T cells differentiate into effector cells (migrating to various tissues, multiplying, and releasing effectors) and memory cells (with prolonged lifespans and self-renewing abilities).
These memory cells include stem-like memory T cells (TSCM), central memory T cells (TCM), and effector memory T cells (TEM), each characterized by specific cell markers.
Next, the authors discuss how glucose, fatty acids, and amino acids feed into T cell energy production. T cell mitochondria influence development, long-term survival, and the response to antigen challenge.
Naïve T cells rely on oxidative phosphorylation (OXPHOS) but switch to aerobic glycolysis to meet the energy demands for rapid proliferation and effector functions when stimulated. Moreover, subsets of memory T cells have distinct metabolic profiles with TCM and TSCM cells depending on OXPHOS and fatty acid oxidation (FAO). In this manner, the control of energy metabolism determines the fate of T cells.
Glucose, via pyruvate, is the primary fuel that supports the differentiation of effector T cells and the survival of memory T cells. FAO supports memory T cell differentiation, with the cells favoring the oxidation of long-chain fatty acids sourced from lipids, glycerol, and cholesterol.
Amino acids are needed for CD8+ T cell proliferation, differentiation, and function, as they support macromolecular biosynthesis, the redox balance within the cells, and post-transcriptional alterations.
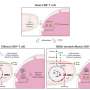
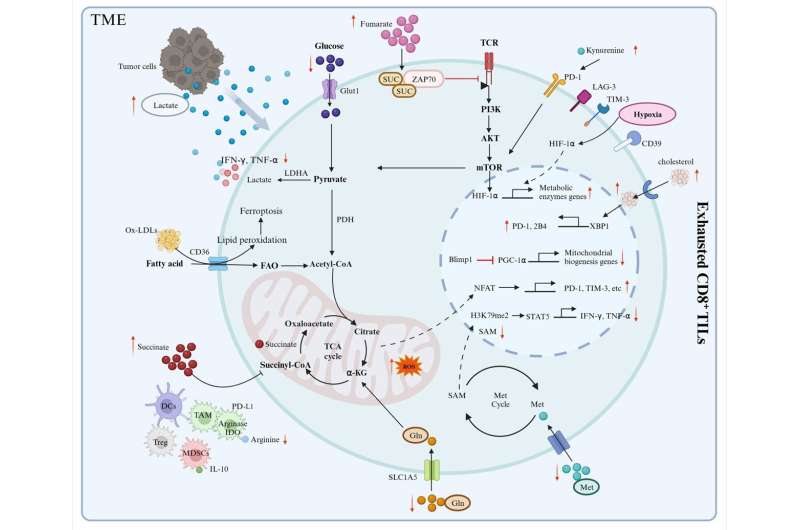
Lastly, the authors discuss how metabolic programs cause T cell malfunctioning. The TME is hostile as tumor cells compete with CD8+ tumor-infiltrating lymphocytes (TILs) for nutrients to feed their growth and metastasis.
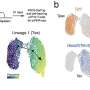
The TME is defined by nutrient deprivation, hypoxia, and immunosuppressive metabolites that exacerbate T cell exhaustion (there are two types: progenitor exhausted T cells and terminally exhausted T cells) and impair anti-tumor activity. Progenitor exhausted T cells rely on mitochondrial FAO and OXPHOS, but terminally exhausted T cells depend on glycolytic metabolism for energy production.
The limited glucose in the TME and intermediates of glucose metabolism can compromise TIL proliferation and function. Furthermore, the switch to OXPHOS yields reactive oxygen species that damage mitochondria. This induces epigenetic remodeling, which in turn, promotes exhaustion.
Similarly, depletion of amino acids in the TME and utilization of fatty acids as an alternative to glucose can cause TIL dysfunction. Oxygen availability plays a role in the viability of TILs.
Aberrant angiogenesis in and around tumors drives hypoxic conditions that induce metabolic dysfunction in TILs, preventing them from eliminating tumor cells. Interestingly, lactate acidifies the TME and impairs T cell effector functions, but it can also enhance T cell biosynthetic capacity via conversion to pyruvate under glucose starvation.
In summary, targeting glucose, amino acid, and lipid metabolism can open novel avenues to treat cancer and improve T cell function.
"Metabolic reprogramming of T cells in the TME induces changes in the epigenetic landscape, influencing T cell differentiation, proliferation, survival, and anti-tumor immunity. Exhausted T cells in tumors exhibit a distinctive feature known as 'epigenetic scarring,' which persists over time, even after antigen clearance and blocks future immune responses. Whether targeted metabolism can clear this 'epigenetic scarring' and potentially delay or reverse T cell exhaustion requires further investigation," concludes Prof. Huang.
More information: Xiaoli Pan et al, Metabolic plasticity of T cell fate decision, Chinese Medical Journal (2023). DOI: 10.1097/CM9.0000000000002989